Abstract
Introduction: Mechanical jaundice (MJ) or bile duct blockage occurs when the bile ducts' patency is impaired, and the bile flow has stopped. One of the main pathogenetic factors developing complications with MJ is immune system imbalance, particularly its phagocytic link. The purpose of the study was to understand neutrophils' functional activity dependence with different blood bilirubin levels in men with mechanical jaundice.
Methods: Forty-seven middle-aged men with mechanical jaundice were divided into three groups depending on the bilirubin levels in their blood: less than 60 μmol/L (n = 10), 60 – 200 μmol/L (n = 20), and more than 200 μmol/L (n = 17). The control group consisted of 50 practically healthy men of the same age. The neutrophils' functional state was assessed using the methods of spontaneous and induced luminol-dependent chemiluminescence of neutrophils.
Results: In the group of patients with mechanical jaundice and a bilirubin level of less than 60 μmol/L, there was an increase in the values of T max spontaneous by 96%, I max spontaneous by 44.81%, S spontaneous by 224.6%, T max induced by 19.9%, I max induced by 13.5%, and S induced by 140.3%. In the group with bilirubin levels from 60 – 200 μmol/L, there was an increase in the values of T max spontaneous by 86.8%, I max spontaneous at 47.7%, S spontaneous at 204.6%, I max induced at 28.3%, S induced at 445%, and activation index at 70%. The group with bilirubin levels more than 200 μmol/L showed an increase in the level of T max spontaneous by 85.9%, I max spontaneous by 53.4%, S spontaneous by 927.3%, I max induced by 28.6%, S induced by 1045%, and activation index by 92.3% compared with the control values. The intergroup differences were found in S spontaneous levels, which were higher in the group with more than 200 μmol/L bilirubin levels compared with the 60 – 200 μmol/L group and less than the 60 μmol/L groups by 216.9% and 237.3% respectively.
Conclusion: The revealed changes characterize the functional activity of neutrophils' increase with an increase in the bilirubin levels in patients with mechanical jaundice.
Introduction
Mechanical jaundice (MJ) or bile duct blockage (code K83.1, according to ICD 10) is a clinical and morphological manifestation complex that occurs when the bile ducts' patency is impaired, and the bile flow from the liver to the duodenum has stopped1. This pathology is detected in 12 — 45% of cases of hepatopancreatoduodenal diseases, mainly in men2, 3. Complications involving MJ include biliary tract disturbances, dysfunctional liver disorders, and numerous systemic disorders4, 5. One of the main pathogenetic factors that develop complications with MJ is immune system imbalance, particularly its phagocytic link6, 7. This is manifested through phagocytosis process violations, changes in interleukin levels, neutrophil degranulation, and oxidative stress development8, 9, 10, 11. Neutrophils' functional activity depends on the intensity of the respiratory burst process, and intracellular metabolism12, 13. The chemiluminescence (ChL) method allows the analysis of the features of a respiratory burst in spontaneous and induced states in various diseases14. An extremely important indicator for patients with MJ is their bilirubin level, which plays a crucial role in the disease progression15. It has been previously shown that the increased levels of serum bilirubin may be the reason for the bactericidal activity of neutrophils' impairment in patients with hyperbilirubinemia16, 17. Studying the neutrophils' functioning mechanisms and their relationship with bilirubin levels allow to identify intracellular targets, which makes it possible to modulate the cells' reactivity. Neutrophil dysfunction's detection can contribute to MJ's differential diagnosis and prognosis. In this regard, our work aimed to study neutrophils' functional activity dependence with the bilirubin levels in men with mechanical jaundice.
Methods
Design of the study
The study used data on men with MJ (n = 47; mean age — 52.02 ± 5.18 years) who were treated at the Research Institute of Medical Problems of the North Krasnoyarsk Science Center. The control group consisted of practically healthy men (n = 50; average age — 48.7 ± 3.9 years) who underwent a routine medical examination. The criteria for inclusion for the clinical and control groups were as follows: male, age – 45–59 years, and informed consent to participate in the study. The criteria for exclusion for the groups included the following: severe somatic diseases, including tuberculosis and HIV infection as well as drug addiction, and refusal to participate in the study. The diagnosis of mechanical jaundice (MJ) is based on (determined by) a combination of clinical and diagnostic signs (pronounced pain syndrome, bilirubin levels, alkaline phosphatase levels, liver enzymes levels, liver ultrasound, etc.) and the existence of cholelithiasis. The patients with MJ were divided into three subgroups depending on the bilirubin levels in blood: less than 60 µmol/L (n = 10), 60–200 µmol/L (n = 20), and more than 200 µmol/L (n = 17)18.
Ethics approval for research
Before beginning the study, the Ethics Committee of the Research Institute of Medical Problems of the North Krasnoyarsk Science Center had approved it (protocol № 7 of November 16, 2012). Ethical principles were observed in accordance with the Helsinki Declaration of the World Medical Association (1964, ed. 2013).
Biochemical measurements
The neutrophils' functional state was assessed at the time of the patients' admission to the hospital and before therapy. Peripheral blood from the ulnar vein was used. The method of neutrophils' spontaneous and induced luminol-dependent chemiluminescence (ChL) assessing was used19, 20. ChL was assessed by the biochemiluminescence analyzer BLM-3607 for 90 minutes. The following parameters were analyzed: the time when the curve reached the maximum level of ChL intensity (T max spontaneous and T max induced), the maximum intensity of the c ChL (I max spontaneous and I max induced), and the square under the curve of spontaneous ChL (S spontaneous) and induced ChL (S induced). Luminol was used as ChL amplifier, and opsonized zymosan served as an inducer of a respiratory burst. We also used the activation index indicator — the ratio of S induced to S spontaneous.
Statistical procedure
Statistical processing of the material was done using Statistica on Windows 8.0 application software package (Stat SoftInc., USA, 2008), and it included methods for determining the proximity of the sample to the normal distribution law (visual-graphical method, Kolmogorov–Smirnov agreement criteria, and Lilliefors and Shapiro–Wilk tests). The Mann–Whitney rank test was also used to analyze the statistical significance of the revealed differences among quantitative data. The critical significance level was assumed to be p < 0.05.
Results
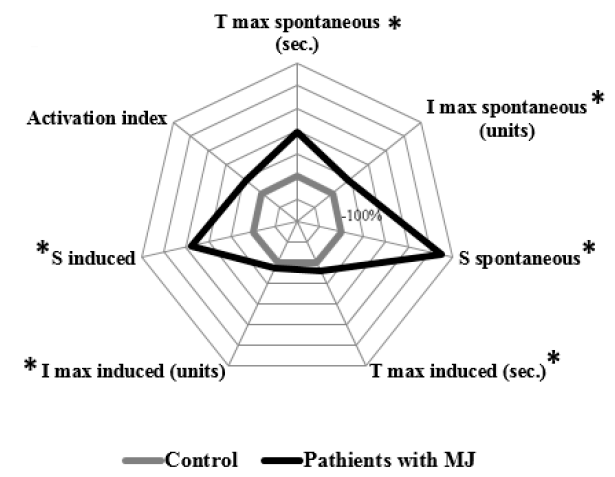
In the group of patients with MJ and bilirubin levels less than 60 µmol/L, there was a statistically significant increase in the values of T max spontaneous by 96% (p = 0.02), I max spontaneous by 44.81% (p = 0.015), S spontaneous by 224.6% (p = 0.015), T max induced by 19.9% (p = 0.04), I max induced by 13.5% (p = 0.01), and S induced by 140.3% (p = 0.015) compared with the control values (Figure 1).
The group of patients with MJ and bilirubin levels from 60 to 200 µmol/L were characterized by a statistically significant increase in T max spontaneous by 86.8% (p < 0.001), I max spontaneous by 47.7% (p < 0.001), S spontaneous by 204,6% (p < 0.001), I max induced by 28.3% (p < 0.001), S induced by 445% (p < 0.001), and activation index by 70% (p < 0.001) compared with the control values (Figure 2).
The group of patients with MJ and bilirubin levels more than 200 µmol/L differed as well as the previous group with an increase in the level of T max spontaneous by 85.9% (p < 0.001), I max spontaneous by 53.4 % (p < 0.001), S spontaneous by 927.3 % (p < 0.001), I max induced by 28.6% (p < 0.001), S induced by 1045 % (p < 0.001), and activation index by 92.3% (p < 0.001) compared with the control values (Figure 3).
The intergroup differences related to S spontaneous, which was higher in the group with bilirubin levels of more than 200 µmol/L compared with the groups with bilirubin levels less than 60 µmol/L by 216.9 % (p = 0.002) and the group with 60–200 µmol/L bilirubin levels by 237.3% (p < 0.001).
Discussion
The concept of respiratory refers to the process of increasing the synthesis of reactive oxygen species (ROS) by phagocytic cells, which occurs either during phagocytosis or under regulatory influences21, 22. Primary and secondary ROS have a moderate bactericidal and regulatory effect and are synthesized in cells during enzymatic or non-enzymatic reactions22, 23, 24, 25, 26. According to the literature, neutrophils' functional activity directly depends on the chemiluminescence's intensity; the higher the chemiluminescence, the greater their functional capacity19. Due to the low quantum yield of spontaneous luminescence, special luminescence acceptors, known as indicators, are actively introduced to sensitize luminescence. The ability of one such indicator, luminol, to interact with both primary and secondary ROS determines its inability to be used to assess particular ROS synthesis levels, but it allows us to integrally characterize the state of the respiratory burst of phagocytic cells27. In our study, an increase in T max was observed with spontaneous and induced chemiluminescence in patients with MJ and bilirubin levels less than 60 µmol/L. This indicator reflects the duration for the development of the maximum activity of ROS synthesis from the moment of antigenic induction of phagocytes' respiratory explosion and depends on the state of cells' metabolic reactions12, 28. Their values, as a rule, decrease in the case of acute infectious and inflammatory diseases when neutrophils are in the activated state and increase in chronic inflammatory processes29, 30, 31. I max spontaneous and induced, reflecting the maximum synthesis of ROS by the cell, also increases at bilirubin levels below 60 µmol/L.
The square under the chemiluminescence curve, which integrally characterizes the entire complex of ROS (S) produced by phagocytes during the study period, changed in a similar way, with the most intense increase in spontaneous chemiluminescence. The group with bilirubin levels of 60–200 µmol/L was characterized by initial changes related to the control, while there was a tendency for a slight decrease in the values of a number of indicators.
The greatest increase was found in the S induced parameter, which characterizes the entire phagocytic pool of ROS, and was confirmed by the increase in activation index in this group. Activation index, which is the ratio of S induced to S spontaneous, shows the presence of intracellular metabolic reserves for the implementation of a respiratory burst32. The study revealed a significant increase regarding this parameter in patients with MJ and 60–200 µmol/L bilirubin levels. The group with MJ and more than 200 µmol/L bilirubin levels also showed an increase in indicators related to the control. However, in this group, there was also a significant increase in the values of S spontaneous compared with the group with lower bilirubin levels.
Bilirubin, belonging to bile pigments, is a product of the catabolism of heme-containing compounds and is mainly considered a negative factor in diseases associated with the liver and biliary tract20. The toxic effect of elevated bilirubin in blood on patients with MJ is manifested through the appearance of foci of necrosis in parenchymal organs, suppression of cellular immune response, and other effects21, 33. The final stage of the pathological process in hepatic parenchyma is the initiation of hypoxic or free radical necrobiosis, subsequently causing cell death1, 2. Despite the available data on the inhibitory ability of bilirubin in relation to neutrophil activity, we found an increase in the total square under the chemiluminescence curve, which characterizes the entire complex of ROS (S spontaneous) produced by phagocytes. This fact can be associated with the benign genesis of the disease, when numerous pathological phenomena can be reversible, in contrast to the tumor process3, 8.
Conclusions
The changes in patients with mechanical jaundice reflect a significant increase in the neutrophils' functional activity in proportion to the bilirubin levels in the blood. These indicators can serve as additional laboratory markers that determine the course of the disease.
Abbreviations
ChL: chemiluminescence
I: intensity
Max: maximum
MJ: mechanical jaundice
ROS: reactive oxygen species
S: square
T: time
Acknowledgments
None
Author’s contributions
Research concept and design: Darenskaya MA, Smirnova OV, Kasparov EV
Funding
The work was carried out within the framework of the state budgetary theme.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Pavlidis
E.T.,
Pavlidis
T.E.,
Pathophysiological consequences of obstructive jaundice and perioperative management. Hepatobiliary Pancreat Dis Int.
2018;
17
(1)
:
17-21
.
View Article PubMed Google Scholar -
Khan
R.S.,
Houlihan
D.D.,
Newsome
P.N.,
Investigation of jaundice. Medicine (Abingdon).
2019;
47
(11)
:
713-7
.
View Article Google Scholar -
Zakaria
A.S.,
Gamil
M.,
Ali Eldin
N.H.,
Mebed
A.H.,
P5: malignant obstructive jaundice; review of 232 Patients. Pan Arab Journal of Oncology..
2019;
12
(3)
:
55
.
-
Papadopoulos
V.,
Filippou
D.,
Manolis
E.,
Mimidis
K.,
Haemostasis impairment in patients with obstructive jaundice. J Gastrointestin Liver Dis.
2007;
16
(2)
:
177-86
.
PubMed Google Scholar -
Huynh
F.,
Usatoff
V.,
Obstructive jaundice. Textbook of SurgeryJohn Wiley & Sons Ltd: Hoboken (NJ); 2019.
View Article Google Scholar -
Hajiyev
J.M.,
Taghiyev
E.G.,
Hajiyev
N.J.,
ogly Hajiyev JM, oglu Taghiyev EG, oglu Hajiyev NJ. Comparative evaluation of interleukin-6 in the liver tissues, bile duct, blood serum and urine in patients with obstructive jaundice of benign etiology. Journal of Experimental and Clinical Surgery.
2016;
9
(1)
:
33-8
.
View Article Google Scholar -
Shevchenko
B.F.,
Zeleniuk
O.V.,
Klenina
I.A.,
Babii
O.M.,
Structural and functional state of the liver in patients with extrahepatic cholestasis of non-tumor genesis. Reports of Morphology.
2019;
25
(4)
:
36-43
.
View Article Google Scholar -
Darenskaya
M.A.,
Smirnova
O.V.,
Gubanov
B.G.,
Semenova
N.V.,
Kolesnikova
L.I.,
Kolesnikov
S.I.,
Lipid peroxidation, antioxidant defense parameters, and dynamics of surgical treatment in men with mechanical jaundice of various origins. AIMS Mol Sci.
2020;
7
(4)
:
374-82
.
View Article Google Scholar -
Darenskaya
M.A.,
Kolesnikov
S.I.,
Rychkova
L.V.,
Grebenkina
L.A.,
Kolesnikova
L.I.,
Oxidative stress and antioxidant defense parameters in different diseases: ethnic aspects. Free Radic Biol Med.
2018;
120
:
60
.
View Article Google Scholar -
Ershova
O.A.,
Bairova
T.A.,
Kolesnikov
S.I.,
Kalyuzhnaya
O.V.,
Darenskaya
M.A.,
Kolesnikova
L.I.,
Oxidative stress and catalase gene. Bull Exp Biol Med.
2016;
161
(3)
:
400-3
.
View Article PubMed Google Scholar -
Darenskaya
M.A.,
Gavrilova
O.A.,
Rychkova
L.V.,
Kravtsova
O.V.,
Grebenkina
L.A.,
Osipova
E.V.,
The assessment of oxidative stress intensity in adolescents with obesity by the integral index. Int J Biom.
2018;
8
(1)
:
37-41
.
View Article Google Scholar -
Proskurnina
E.V.,
Dzhatdoeva
A.A.,
Lobichenko
E.N.,
Shalina
R.I.,
Vladimirov
Y.A.,
Chemiliminescence determination of lipid hydroperoxides in biological fluids. J Anal Chem.
2017;
72
(7)
:
751-5
.
View Article Google Scholar -
Smirnova
E.V.,
Krasnova
T.N.,
Proskurnina
E.V.,
Mukhin
N.A.,
Role of neutrophil dysfunction in the pathogenesis of systemic lupus erythematosus. Ter Arkh.
2017;
89
(12)
:
110-3
.
View Article PubMed Google Scholar -
Kolesnikova
L.I.,
Darenskaya
M.A.,
Kolesnikov
S.I.,
Free radical oxidation: pathophysiologist's view. Byulleten sibirskoy meditsiny.
2017;
16
(4)
:
16-29
.
View Article Google Scholar -
Uemura
S.,
Higuchi
R.,
Yazawa
T.,
Izumo
W.,
Otsubo
T.,
Yamamoto
M.,
Level of total bilirubin in the bile of the future remnant liver of patients with obstructive jaundice undergoing hepatectomy predicts postoperative liver failure. J Hepatobiliary Pancreat Sci.
2020;
27
(9)
:
614-21
.
View Article PubMed Google Scholar -
Arai
T.,
Yoshikai
Y.,
Kamiya
J.,
Nagino
M.,
Uesaka
K.,
Yuasa
N.,
Bilirubin impairs bactericidal activity of neutrophils through an antioxidant mechanism in vitro. J Surg Res.
2001;
96
(1)
:
107-13
.
View Article PubMed Google Scholar -
Vagholkar
K.,
Obstructive Jaundice: understanding the pathophysiology. International Journal of Surgery and Medicine.
2020;
6
(4)
:
26-31
.
View Article Google Scholar -
Galperin
E.A.,
Momunova
O.N.,
Classification of the severity of obstructive jaundice. Surgery. Journal them. N.I. Pirogov.
2014;
1
:
5-9
.
-
Lippa
S.,
De Sole
P.,
Meucci
E.,
Littarru
G.P.,
De Francisci
G.,
Magalini
S.I.,
Effect of general anesthetics on human granulocyte chemiluminescence. Experientia.
1983;
39
(12)
:
1386-8
.
View Article PubMed Google Scholar -
Savchenko
A.A.,
Kudryavtsev
I.V.,
Borisov
A.G.,
Assessment methods and the role of respiratory burst in the pathogenesis of infectious and inflammatory diseases. Infektsiia Immun.
2017;
7
(4)
:
327-40
.
View Article Google Scholar -
Winterbourn
C.C.,
Kettle
A.J.,
Hampton
M.B.,
Reactive oxygen species and neutrophil function. Annu Rev Biochem.
2016;
85
(1)
:
765-92
.
View Article PubMed Google Scholar -
Darenskaya
M.A.,
Grebenkina
L.A.,
Sholokhov
L.F.,
Rashidova
M.A.,
Semenova
N.V.,
Kolesnikov
S.I.,
Kolesnikova
L.I.,
Lipid peroxidation activity in women with chronic viral hepatitis. Free Radical Biology & Medicine.
2016;
100
(S)
:
S192
.
View Article Google Scholar -
Kolesnikova
L.I.,
Kolesnikov
S.I.,
Darenskaya
M.A.,
Grebenkina
L.A.,
Nikitina
O.A.,
Lazareva
L.M.,
Activity of LPO processes in women with polycystic ovarian syndrome and infertility. Bull Exp Biol Med.
2017;
162
(3)
:
320-2
.
View Article PubMed Google Scholar -
Kolesnikova
L.I.,
Rychkova
L.V.,
Kolesnikova
L.R.,
Darenskaya
M.A.,
Natyaganova
L.V.,
Grebenkina
L.A.,
Coupling of lipoperoxidation reactions with changes in arterial blood pressure in hypertensive ISIAH rats under conditions of chronic stress. Bull Exp Biol Med.
2018;
164
(6)
:
712-5
.
View Article PubMed Google Scholar -
Kolesnikova
L.I.,
Kolesnikov
S.I.,
Korytov
L.I.,
Suslikova
M.I.,
Darenskaya
M.A.,
Grebenkina
L.A.,
Oxidative stress as a mechanism of reduced glucose absorption under conditions of immobilization stress. Bull Exp Biol Med.
2017;
164
(2)
:
132-5
.
View Article PubMed Google Scholar -
Kolesnikova
L.I.,
Madaeva
I.M.,
Semenova
N.V.,
Vlasov
B.Y.,
Grebenkina
L.A.,
Darenskaya
M.A.,
Antioxidant potential of the blood in men with obstructive sleep breathing disorders. Bull Exp Biol Med.
2013;
154
(6)
:
731-3
.
View Article PubMed Google Scholar -
Bedouhène
S.,
Moulti-Mati
F.,
Hurtado-Nedelec
M.,
Dang
P.M.,
El-Benna
J.,
Luminol-amplified chemiluminescence detects mainly superoxide anion produced by human neutrophils. Am J Blood Res.
2017;
7
(4)
:
41-8
.
PubMed Google Scholar -
Nakamura
M.,
Umemura
K.,
Hiramatsu
M.,
Oishi
H.,
Maekawa
M.,
Efficacy of stress measurements using salivary ultra-weak chemiluminescence. Traumatology.
2019;
67
(4)
:
2
.
-
Davis
R.W.,
Snyder
E.,
Miller
J.,
Carter
S.,
Houser
C.,
Klampatsa
A.,
Luminol chemiluminescence reports photodynamic therapy-generated neutrophil activity in vivo and serves as a biomarker of therapeutic efficacy. Photochem Photobiol.
2019;
95
(1)
:
430-8
.
View Article PubMed Google Scholar -
Saqib
M.,
Qi
L.,
Hui
P.,
Nsabimana
A.,
Halawa
M.I.,
Zhang
W.,
Development of luminol-N-hydroxyphthalimide chemiluminescence system for highly selective and sensitive detection of superoxide dismutase, uric acid and Co2. Biosens Bioelectron.
2018;
99
:
519-24
.
View Article PubMed Google Scholar -
Piknova
B.,
Park
J.W.,
Cassel
K.S.,
Gilliard
C.N.,
Schechter
A.N.,
Measuring nitrite and nitrate, metabolites in the nitric oxide pathway, in biological materials using the chemiluminescence method. J Vis Exp.
2016;
118
(118)
:
e54879
.
View Article PubMed Google Scholar -
Su
Y.,
Song
H.,
Lv
Y.,
Recent advances in chemiluminescence for reactive oxygen species sensing and imaging analysis. Microchem J.
2019;
146
:
83-97
.
View Article Google Scholar -
Redza-Dutordoir
M.,
Averill-Bates
D.A.,
Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta.
2016;
1863
(12)
:
2977-92
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 8 No 6 (2021)
Page No.: 4417-4422
Published on: 2021-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5574 times
- Download downloaded - 1768 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress





