Abstract
Introduction: Bubonium graveolens is used in traditional pharmacopoeia against imbalances of the gastrointestinal tract, cephalic pains, and bronchitis, and as an anti-inflammatory agent.
Methods: We have investigated the analgesic and anti-inflammatory activities of the aqueous extract of Bubonium graveolens in male mice of strain NMRI Albinos, weighing between 22 g and 38 g. Acetic acid was used to induce writhes in the mice and inflammation of paw edema.
Results: Evaluation of the analgesic activity showed that the aqueous extract at 150 mg/kg of the plant induced a decrease in the number of abdominal cramps caused by 1% acetic acid. The aqueous extract of the plant had an analgesic effect almost equal to that of Diclofenac; in fact, the latter caused a pain inhibition of 49 ± 1.1% while Bubonium graveolens caused a pain inhibition of 49.6 ± 2.1%, at the concentration of 150 mg/kg. Evaluation of the percentage of inhibition showed that the aqueous extract of Bubonium graveolens had a better anti-inflammatory activity compared to Diclofenac sodium during the treatment duration (69.57% — 56.52% at 60 min; 71.43% — 50.00% at 120 min, and 75.00% — 66.67% at 180 min).
Conclusion: The results of this research indicate that Bubonium graveolens inhibits inflammation and could explain its effective use in traditional medicine.
INTRODUCTION
Bubonium graveolens is a medicinal plant growing in arid and semi-arid climates. It stretches from the north of Africa to the desert of Central Asia but is widely represented in southwestern Algeria and southern Morocco1, 2. It is considered as one of the most important Saharan plants, used specifically for its medicinal and aromatic properties3, 4. It is known locally as 'Tafss'1.
Bubonium graveolens is used in the treatment of gastrointestinal disorders, fever, cephalic, dietary, and bronchitis, as well as an anti-inflammatory agent5, 6, 7. Recently it was used as a botanical fungicide8, 9 and was reported to possess antimicrobial and hypoglycemic activities10, 11.
According to literature review, a few studies have been done on these species and have shown this plant to be rich in monoterpenes12, flavonoids13, sesquiterpenes14, and essential oils (43 compounds have been identified; the latter are present in the leaves and flowers)1. The essential oils of Bubonium graveolens contain mainly oxygenated monoterpenes (37.6% in leaves, and 60.3% in flowers), with 1,8-cinoeole (21.5%) as the main constituent in the flowers1. The other major components are cis-chrysanthenyl acetate (44.30%), cis-8-acetoxychrysanthenyl acetate (33.70%), and τ-muurolol (6.51%)15.
The aim of the study herein is to evaluate the pharmacological properties of the aqueous extract of Bubonium graveolens to provide a scientific basis for the empirical use of this plant in traditional pharmacopoeia. Therefore, justification of the potential use of Bubonium graveolens as an analgesic will be assessed and verified.
MATERIALS - METHODS
Plant material
Aerial parts of Bubonium graveolens were collected during flowering in south-western Algeria (March 2018), and identified by the National Agency of Nature Protection (ANN), Bechar, Algeria. Botanical identification and voucher specimen are conserved in the Medicinal Plant Encyclopedia Herbarium of bioactive molecules and Chiral Separation Laboratory (BMCS Lab) under accession number MPE11-7-E3.
Preparation of the extract
The aqueous extract was prepared by Soxhlet extraction of 25 grams of powder in 150 mL of distilled water for 6 hours. After decantation, the supernatant was filtered and the filtrate was evaporated under vacuum in a rotary evaporator. An aqueous extract of various concentrations (50 mg/kg, 100 mg/kg, 150 mg/kg, 200 mg/kg, 750 mg/kg, 1000 mg/kg, and 2000 mg/kg) were prepared.
Apparatus
Plethysmometer (LE 7500 plethysmometer, Barcelona, SPAIN) was used for measuring the volume of the mouse paw.
Reagents
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Animals
Permission was obtained from the Committee of use of animal experiments (Approval # 943-19). The study was carried out on mice NMRI Albinos, 100% males, from the breeding laboratory of the Pasteur Institute of Algeria. Their weight was between 22 and 38 g. Mice were randomly housed in plastic cages with controlled temperature (25°C) and under a 12 h light–dark cycle. Animals benefited from an adaptation period before use. They were fed and maintained under standard procedures, with access to water and standard food (Bovin fattening). The mice were with an empty stomach for 17 hours before each experiment16.
Toxicity test
For toxicity testing, 8 batches of 6 mice were used for testing the aqueous extract of Bubonium graveolens. Doses administered were as follows: 50, 100, 150, 200, 750, 1000 and 2000 mg/kg of the aqueous extract. They were administered with a physiological solution at the rate of 10 μl/g of mouse body weight; the control batch was administered with physiological water at a rate of 10 μl/g.
The mice were respectively dosed intraperitoneally (IP) with aqueous extract or physiological water, based on body weight. They were then observed for 2 hours to record immediate signs and behavior following intoxication, and monitored against the control group. After the 2 hours, the mice were given food and water, followed by two observation periods (one for 24 hours and one for 48 hours).
Study of analgesic activity
Test Writhing
The method used is similar to that described by Koster et al.17. We studied analgesic activity in mice using a pain reaction, which is caused in mice by intraperitoneal (IP) injection of acetic acid 1%18.
Five groups of six mices were constituted:
Control batch: The mice of this batch receive physiological saline 30 minutes before the injection of acetic acid (1%) by IP according to body weight.
Batch reference: The animals of this batch were treated by subcutaneous injection (SC) of Diclofenac sodium, at 30 minutes before the IP injection of acetic acid (1%).
Test batch: The animals receive IP administration of the extracts at these concentrations (50 mg/kg, 100 mg/kg, 150 mg/kg, 175 mg/kg), at 30 minutes before the injection of acetic acid (1%), according to body weight.
Five minutes after the injection of acetic acid, we counted the number of cramps in each mouse for 20 minutes. The percentage of inhibition of the tested products was evaluated by the determination of the mean of cramps, calculated according to the formula:
A: represents the average cramp of the mice of the control group.
B: represents the mean cramps of the mice of the treated lots.
Anti-Inflammatory activity
The anti-inflammatory activity study was evaluated by the method of inhibition of 1% formalin-induced mouse paw edema. The mice before each experiment were with an empty stomach for 17 hours, at which time inflammation is induced by injecting formalin into the plantar arch of the left mouse paw19.
Measurements of the volumes of the right hind paw of each mouse were performed prior to induction of edema and every 1 h, 2 h, 3 h, 4 h and 5 h after the formalin injection. Half an hour before the formalin injection, the different lots of mice received the different treatments:
A control group of 6 mice treated IP with physiological water, according to body weight.
A reference batch of 6 mice treated by IP with Diclofenac sodium (20 µl).
A batch of 6 mice treated with the extract IP with the plant extract Bubonium graveolens at a dose of 150 mg/kg, according to body weight.
The anti-inflammatory activity of the products was tested and its evolution was estimated by the determination of the average percentages of inhibition of the edema, calculated according to the formula:
V0: represents the volume of the paw at t = 0 (before injection of formalin).
Vt: represents the volume of the paw at any time t.
Statistical analysis
The obtained results were subjected to an analysis of the variance (ANOVA) at a probability level of P < 0.05, and (n) represents the number of mice in each group.
RESULTS
Toxicity
Immediate signs of change, intoxication, and sudden death (in comparison with control animals) were followed for 2 hours after IP administration of the aqueous extract. At 24 h and then 48 h later, we again observed the mice to determine the delayed effects of taking different doses of the aqueous extract of Bubonium graveolens. Intra-peritoneal administration causes changes in the physical activity and behavior of the mice. Table 1 summarizes the immediate signs recorded during this experiment.
| Product | Doses (mg/kg) | Symptoms | Mortality | ||
| Hypoactivity | Drowsiness | Tachycardia | |||
| Aqueous extract | 50 | - | - | - | 0 |
| 100 | - | - | - | 0 | |
| 150 | - | - | - | 0 | |
| 200 | + | + | + | 0 | |
| 750 | ++ | ++ | ++ | 0 | |
| 1000 | +++ | +++ | +++ | 0 | |
| 2000 | +++ | +++ | +++ | 0 | |
| - : No sign; + :High sign | |||||
The observed results show that doses of 50 to 150 mg/kg did not cause any noticeable change in the behavior or activity of the mice. On the other hand, it should be noted that the animals receiving doses of 200, 750, 1000 and 2000mg/kg showed strong signs of hypo-activity, drowsiness and tachycardia, with no mortality for the duration of study.
Writhing test
The acetic acid induced writhing test is a simple and commonly used method for screening analgesic drugs. The administration of acetic acid is responsible for the release of endogenous substances which are supposed to excite the nerve endings, thereby causing the pain20.
The following table (Table 2) gives us a representation of the analgesic activity of the aqueous extract of Bubonium graveolens.
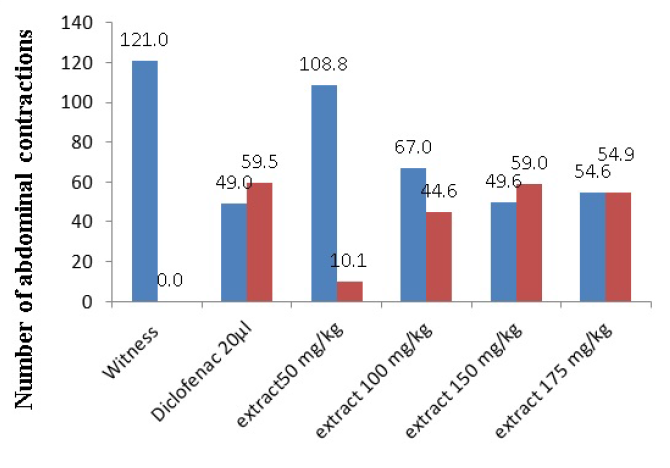
| Organs and doses (mg/kg) | Number of abdominal contractions | Percent inhibition (%) |
| Witness | 121 ± 0.7 | - |
| Diclofenac (20 µl) | 49 ± 1.1 | 59.50 |
| Excerpts | ||
| 50 | 108.8 ± 0.4 | 10.08 |
| 100 | 67 ± 2.1 | 44.62 |
| 150 | 49.6 ± 2.1 | 59.00 |
| 175 | 54.6 ± 1 | 54.87 |
The control group which received physiological saline showed writhing after the intraperitoneal injection of 1% acetic acid; the average number of abdominal contractions were 121 ± 0.7, with a percentage inhibition of 0% over a duration of 20 minutes.
Subcutaneous administration of Diclofenac sodium (20 μl) followed by injection of acetic acid yielded an average number of abdominal contractions of 49 ± 1.1, which was equivalent to a percentage inhibition of 59.50% (p ˂ 0.05, n = 5).
Administration by IP route of the aqueous extract of Bubonium graveolens in progressive doses (i.e. 50, 100, 175 mg/kg) yielded the following results: for 50 mg/kg dose, the mean number of abdominal contractions was 108.8 ± 0.4, i.e. percentage of inhibition of 10.08%; for 100 mg/kg dose, the mean number of abdominal contractions was 67 ± 2.1, i.e. percentage inhibition of 44.62%; for 175 mg/kg dose, the mean number of abdominal contractions was 54.6 ± 1, i.e. percentage of inhibition was 54.87% (p ˂ 0.05, n = 5) (Table 2 and Figure 1).
Evaluation of anti-inflammatory activity
Assessment of the anti-inflammatory activity of the aqueous extract was carried out by measuring the volume of edema induced by formalin in three lots of 6 mice (control, test and reference). The results are represented in the form of a curve showing the evolution of the volume of the edema as a function of time (Figure 3). The injection of formalin at the hind paw of the mice causes a progressive increase of the edema with a maximal volume after 3 hours21. Formalin causes local inflammation when injected into the fascia of the sole of the foot22 as well as carrageenan23.
Intraperitoneal administration of Diclofenac sodium at 20 μl prevents the increase in the volume of the mouse paw. Indeed, mouse paw volume after 30 min was 0.073 ± 0.019, after 60 min was 0.067 ± 0.020, after 120 min was 0.062 ± 0.016, and after 180 min was 0.050 ± 0.003.
The dose of 150 mg/kg of the aqueous extract of Bubonium graveolens, administered IP, importantly prevented the inflammatory process. Indeed, the increase in the volume of the mouse paw was 0.075 ± 0.031, 0.060 ± 0.052, 0.055 ± 0.036, and 0.053 ± 0.022 after 30, 60, 120, and 180 min, respectively.
The following figure gives a representation of the percent inhibition of mouse left leg edema induced by formalin (1%) and after administration of the aqueous extract of Bubonium graveolens and Diclofenac.
The anti-inflammatory effect of the aqueous extract of Bubonium graveolens has been evaluated in the present work. The results obtained show that the aqueous extract has an anti-inflammatory activity significant to that of Diclofenac sodium.
The results obtained from the anti-inflammatory tests show that the aqueous extracts of Bubonium graveolens in our study appreciably reduce formalin-induced edema.
DISCUSSION
The results of the evaluation of the analgesic activity allowed us to note that the control mice developed a large number of cramps (121 cramps for 20 minutes) after administration of acetic acid. The mice that were treated with Diclofenac developed a reduced number of cramps compared to the control group; this was confirmed by the observed percentage of protection (59.5% inhibition). Conversely, mice that were treated with our extract developed a reduced number of cramps compared to the control mice. In fact, the percentages of protection against cramps calculated for the different products show a relative superiority of the Bubonium graveolens extract, namely for 150 mg/kg dose (59% inhibition), followed by 175 mg/kg dose (54% inhibition), and 100 mg/kg dose (44% inhibition). The results obtained from the analgesic test show that our aqueous extract appreciably reduces the number of abdominal contractions induced by acetic acid. The number of abdominal contractions is comparable to that of Diclofenac. This shows that our plant has a very robust analgesic activity.
For the study of the biological activities of the extracts of Bubonium graveolens, we reviewed the existing scientific literature on this plant. Phytochemical studies were carried out on the different parts of our plant. These studies made it possible to characterize the presence of flavonoids +, saponosides ++, tannins +, steroids +, unsaturated sterols +++, terpenes +++, and cardenolides + 24. The richness of the aqueous extract of our plant in terms of the different chemical constituents contribute to its analgesic activity.
Our goal was to find an anti-inflammatory agent and this goal was reached. Unfortunately, we were not able to determine the structure of these compounds.
Significant results obtained during this study of the aqueous extract of Bubonium graveolens demonstrated remarkable analgesic properties. These results constitute a scientific basis that justifies the traditional use of Bubonium graveolens in the management of pathologies with an inflammatory component.
CONCLUSION
The anti-inflammatory effect of aqueous extracts of Bubonium graveolens at a dose of 150 mg/kg has been evaluated in the present study. The results obtained show that the aqueous extract has an anti-inflammatory activity significant to that of Diclofenac sodium. The results obtained from the anti-inflammatory tests show that our aqueous extract of Bubonium graveolens appreciably reduce formalin-induced edema.
The analgesic activity of the aqueous extract of Bubonium graveolens at a dose of 150 mg/kg caused a percentage of inhibition very near to that of Diclofenac (49% and 49.60%, respectively). The aqueous extract of Bubonium graveolens show an anti-inflammatory activity in the test of edema. In conclusion, the results of the present study provide evidence for the anti-inflammatory and analgesic activities of Bubonium graveolens growing in Algeria, and could explain the benefits of the traditional use of this plant.
ABBREVIATIONS
IP: intraperitoneal
NMRI: Naval Medical Research Institute
SC: subcutaneeous
μm: Microliter
Acknowledgments
The authors are grateful to Mr A. Benabdelhakem (expert botanist, ANN, Bechar) for the identification of plant.
Author’s contributions
FK and AOEK (Co director of project): performed the significant contributions to conceptualization and design; and the acquisition, analysis, and interpretation of the data. BN and SK: performed the drafting of the article and critical revision for important intellectual content. All the authors approved the final version of the manuscript to be published.
Funding
This work is supported by the General Direction of Scientific Research of Algeria (DGRSDT) as part of Research project (PRFU) N°: B00L01UN080120190002.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Cheriti A, Saad A, Belboukhari N, Ghezali S. The essential oil composition of Bubonium graveolens (Forssk) maire from the Algerian Sahara. J Flavour and Fragrance.
2007;
22
:
286-288
.
View Article Google Scholar -
Aici D, Cheriti A, Bourmita Y, Belboukhari N. Antimicrobial activity of essential oils of Bubonium graveolens (forssk.) and Anvillea radiata (coss.). Phyto Chem Bio Sub J.
2013;
7
:
116-119
.
-
Znini M, Cristofari G, Majidi L, Ansari A, Bouyanzer A, Paolini J, et al. Green approach to corrosion inhibition of mild steel by essential oil leaves of Asteriscus graveolens (Forssk.) in sulphuric acid medium. Int J Electrochem Sci.
2012;
7
:
3959-3981
.
-
Said ME, Bombarda I, Naubron JV, Vanloot P, Jean M, Cheriti A, et al. Isolation of the major chiral compounds from Bubonium graveolens essential oil by HPLC and absolute configuration determination by VCD. Chirality.
2017;
29
:
70-79
.
View Article PubMed Google Scholar -
Messaoudi R, cheriti A, bourmita Y. Bioassay-guided isolation of the major compound with antioxidant activity from the algerian medicinal plant bubonium graveolens. J Asian J Pharm Clin Res.
2018 ;
11
:
424-426
.
View Article Google Scholar -
Melekmi N, Saad A, Belboukhari N, Cheriti A. Antimicrobial activity of the essential oil of Bubonium graveolens. Annales de l'université de Bechar.
2006;
2
:
22-26
.
-
Boulenouar N, Marouf A, Cheriti A, Belboukhari N. Medicinal plants extracts as source of antifungal agents against Fusarium oxysporum f. Sp. Albedinis. J Agr Sci Tech.
2012;
14
:
659-669
.
-
Mebarki L, kaid harche M, benlarbi, kasmi H, Matrouine M. Bubonium graveolens extracts for controlling Fusarium oxysporum f. sp. albedinis. J Romanian Biotechnological Letters.
2015;
20
:
10026-10035
.
-
Akssira M, Mellouki F, Salhi A, Alilou H, Saouf A, Hanbali F, et al. Naupliolide, a sesquiterpene lactone with a novel tetracyclic skeleton from Nauplius graveolens subsp. : Odorus, Tetrahedron Lett.
2006;
47
:
6719-6721
.
View Article Google Scholar -
Ramadane F, Belboukhari N, Cheriti A, Zaouani M. Evaluation de la toxicité de deux Astéraceae du Sahara Algérien launaea arbonescens et Bubonium gravoelens. Annales de l'université de Béchar.
;
200
(4)
:
1112-6604
.
-
Ramdane F, Essid R, Mkadmini K, Hammami M, Fares N, Mahammed MH, et al. Phytochemical composition and biological activities of Astericus graveolens (Forssk) extracts. J Process Biochem.
2017;
56
:
186-192
.
View Article Google Scholar -
Chaib F, Allali H, Bennaceur M, Flamini G. Chemical composition and antimicrobial activity of essential oils from the aerial parts of Asteriscus graveolens (Forssk.) less. And Pulicaria incise (Lam.) DC: Two Asteraceae herbs growing wild in the Hoggar. J Chem Biodiver.
2017;
14
:
1700092
.
View Article PubMed Google Scholar -
Ahmed AA, Ishak MS, Micheal HN, El-Ansari MA, El-Sissi HI. Flavonoids of Asteriscus graveolens. J Nat Prod.
1991;
54
:
1092-1093
.
View Article Google Scholar -
Triana J, Eiroa JL, Morales M, Perez FJ, Brouard I, Quintana J, et al. Sesquiterpenoids isolated from two species of the Asteriscus alliance. J Nat Prod.
2016;
79
:
1292-1297
.
View Article PubMed Google Scholar -
Aouissi H, Gourine N, Wang H Xiaochun C, Bombardal Boudjeniba M ,Yousf M. Chemical composition, antioxidative, antimicrobial and anti-cancer activities of Asteriscus graveolens (Forssk) essential oil. J Oriental Pharmacy and Experimental Medicine.
2018;
18
:
135-142
.
View Article Google Scholar -
Ouédraogo N, et al. Etude des activités anti-inflammatoire, analgésique et antipyrétique des décoctés aqueux des feuilles et des racines de Pterocarpus erinaceus Poir. (Fabaceae). J Phytothérapie.
2012;
10
:
286-292
.
View Article Google Scholar -
Koster
R.,
Anderson
M.,
Beer
E.J.,
Acetic acid for analgesic screening. Fed. Proc.
1959;
18
:
412-417
.
-
Rahmani S, Belboukhari N, Sekkoum K, Cheriti A. Evaluation de l'activité anti-inflammatoire d'extraits aqueux de feuilles limoniastrum feei (plumbaginacea). J Algerian journal of arid environment.
2016 ;
1
:
80-86
.
-
Winter
C.A.,
Risley
E.A.,
Nuss
G.W.,
Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med.
1962;
111
:
544-547
.
View Article PubMed Google Scholar -
Nirmal SM, Abdur R, Mohammad AA. Analgesic and Antipyretic Activities of Methanol Extract and Its Fraction from the Root of Schoenoplectus grossus. J Evid Based Complement Alternat Med.
2016;
2016
:
3820704
.
View Article PubMed Google Scholar -
Singla AK, Pathak K. Topical antiinflammatory effects of Euphorbia prostrata on carrageenan-induced footpad oedema in mice. Journal of Ethnopharmacology.
1990;
29
:
291-294
.
View Article Google Scholar -
Sen T, Nag CAK. Antiinflammatory evaluation of Pluchea indica root extract. J of Ethnopharmacology.
1991;
33
:
135-141
.
View Article Google Scholar -
Ossipov MH, Kovelowski C, Porreca F. The increase in morphine antinoceptive potency produced by carrageenan-induced hindpaw inflammation is blocked by nalttrindole, a selective delta-opiod antagonist. Neuroscience Letter.
1995;
184
:
173-176
.
View Article Google Scholar -
Haddouchi F, Chaouche TM, Halla N. Screening phytochimique, activités antioxydantes et pouvoir hémolytique de quatre plantes sahariennes dʼAlgérie. Phytothérapie.
2016;
14
:
1-9
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 9 (2020)
Page No.: 4002-4009
Published on: 2020-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8559 times
- Download PDF downloaded - 2038 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress






