In vitro anti-oxidant and in vivo anti-inflammatory activity determination of the methanolic leaves extract of Millettia pachycarpa
Abstract
Objective: Millettia pachycarpa is a member of the Fabacea family and our object has been set to examine the anti-oxidant and anti-inflammatory endeavor of this plant. This plant has been reported as potential anti-inflammatory agent previously.
Materials and Method: DPPH free radical scavenging and reducing power assay was carried out to assess the electron donating capability of the methanolic crude extract of the specimen. A quantitative analysis has been made to measure phenolic and flavonoid content. The anti-inflammatory potentiality of methanolic extract of M. pachycarpa has been determined via carrageenan-induced paw edema assay in rats.
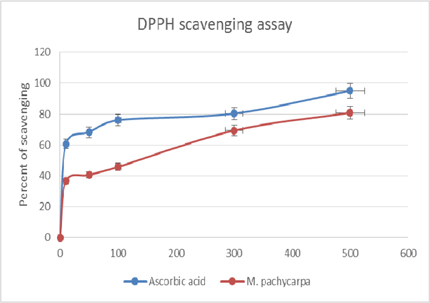
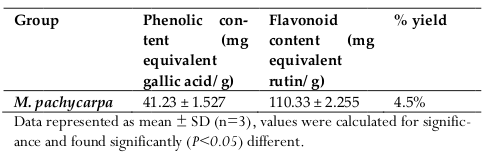
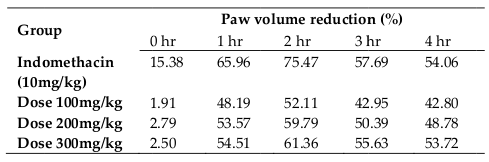
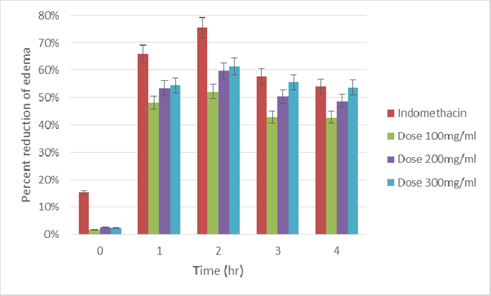
Results: The IC50 value estimated was, 196.47 ± 2.061 µg/ml, which is a very much promising result. From quantitative assessment, sample extract reported very good number of phenolic and flavonoid measurement, 41.23 ± 1.527 mg equivalent gallic acid per gram and 110.33 ± 2.255 mg equivalent rutin per gram, respectively. The studied specimen showed expected dose dependent result in anti-inflammatory assay. At the dose of 300 mg/kg the extract showed maximum inhibitory effect (61.36% at 2 hour; P <0.001) on paw swelling due to Carrageenan injection.
Conclusion: This finding have proved that methanolic extract of M. pachycarpa possess potent anti-inflammatory activity possibly due to presence of good quantity flavonoid and phenolic content, also have good anti-oxidant property.
Introduction
In contemporary medication, even though now we have the supply to enhance synthesis of medicines in a lab, plants are still contributing in health care. However, medicinal plants acquire a great concentration towards them, due to their prolonged use in folk drugs as good as their prophylactic residences, especially in developing countries. Naturally plants have antioxidant activities, and it is supposed that they are been investigated for other pharmacological properties based on these components. They have developed this property as their natural chemical materials, they are very robust to prevent the damaging techniques triggered by oxidative stress Zengin G, 2011. Despite the fact that the toxicity profile of medicinal plants must be evaluated utterly, but it is authorized to all that herbal medicines are safer than their artificial alternative Oluyemi et al., 2007Vongtau et al., 2005.
Oxidation is a process of manufacturing essential energy in living organisms. In that pathway of aerobic metabolism, distinctive reactive oxygen species (ROS) or reactive nitrogen species (RNS) are shaped as intermediates. ROS and RNS comprise free radicals that oxidize the membrane lipids. On this approach, one of a kind cellular additive like proteins, lipids, nucleosides and nucleotides are damaged Samad et al., 2013. Hence, different sickness conditions appear likely, age-associated degenerative psychological disorders, atherosclerosis, cirrhosis, cancer, arthritis, diabetes, hemorrhagic shock, and so forth Gülçin et al., 2010. Each residing organism, a primarily human body has an inherent mechanism that can avert oxidation as well as the anti-mutagenic, anti-carcinogenic, and anti-aging property Göçer and Gülçin, 2011Gülçin, 2011. This mechanism concerned about dietderived antioxidants such as Vitamin E, carotenoids, ascorbic acid, and polyphenols. For this reason, a discovery of average antioxidants derived from food sources as well as other vegetation are receiving much concentrationOzsoy et al., 2009. Antioxidants neutralize or deactivate free radicals, more commonly earlier than they could interact with or pursuits into organic cells Pereira et al.,2012. Up to date interest is developing noticeably towards naturally happening antioxidants to make use of them in pharmaceutical products, considering that they have got multitude and magnitude of anti-oxidant potency that can correct ionic imbalance caused by free radicals Djeridane et al., 2006Wannes WA, 2010.
It is permitted that in an oxidative stress, ROS plays a principal function within the pathogenesis of inflammation Aruoma, 1998Kris-Etherton et al., 2004. Inflammation is a protective mechanism, which took place when the body responds to a kind of stimuli like infections, irritants or different cellular and tissue damages (Oyedapo et al., 2008). The mechanism of inflammation is attributed with the aid of the release of ROS from activated immune procedure, neutrophils and macrophages. Additionally, ROS proliferate inflammation through a stimulating release of cytokines comparable to interleukin-1, TNF-α, and interferon-γ, which stimulate extra neutrophils and macrophages. That is why free radicals are most important mediators, which provoke inflammatory process and their neutralization by antioxidants and radical scavengers can cut back inflammation Delaporte et al., 2002Geronikaki and Gavalas, 2006. At present available anti-inflammatory drugs, inhibit the product from cyclooxygenase (COX) enzymes, COX-1 and COX-2, which includes prostaglandins and thromboxane, common inflammatory mediators Dinarello, 2010. Both steroidal and Non-steroidal antiinflammatory drugs (NSAID’s), plays their role as a cure of irritation or inflammation through inhibiting these two enzyme. Long-term use of these medications results in gastric erosions and duodenal ulcers as well as renal toxicity. Medicinal herbs are new objectives to search out new chemical substances that would have better therapeutic results with low toxic profile.
Millettiapachycarpa belongs to the family of Fabaceae and in a certain community, it is used as fish poison as well as a traditional medicine of multipurpose. It is encouraged in the dietary menu for LDL lowering because it includes phytosterol and ß-sterol, also prescribed as prophylaxis of osteoarthritis mediated cartilage degeneration H, 1996Racette et al., 2009RC, 2010Tattersfield et al., 1940Wu et al., 2009. Millettiapachycarpa has already been suggested as potent antiinflammatory extract Chowdhury A, 2013 from in vitro analysis, as good as this plant is included with tremendous cytotoxic, anti-coagulant and mosquitocidal property Jainul, 2014Jainul MA, 2013K, 2011. Nevertheless, M. pachycarpa yet not been studied in vivo for anti-inflammatory activity determination. Based on the in vitro result published earlier, an in vivo anti-inflammatory study have been attempted with methanolic extracts of leaves of M. pachycarpa. Furthermore, we have also evaluated the primary antioxidant scavenging property of this extract.
Material and Methods
Plant collection and extraction
The plant was collected from Chittagong hill tracts and was identified by Dr. Sheikh Bakhtiar Uddin, Taxonomist, University of Chittagong. A sample specimen was also submitted to Forest Research Institute Chittagong. The leaves of M. pachycarpa was dried at room temperature, powdered and extracted using methanol (80%) Brand-Williams et al., 1995Sakat SS, 2010. Extract was filtered through vacuum filter and then concentrated, the concentrated crude extract was preserved for further use. The percent yield extract was 4.5%.s
DPPH free radical scavenging activity testing
The free radical scavenging capacity of the methanolic extract of M. pachycarpa was measured in vitro by 2, 2 - diphenyl- 1- picrylhydrazyl (DPPH) assay following the system described earlier (Brand-Williams et al.,1995; Bursal and Gülçin, 2011) with minor modification. The parent solution was all set making use of 24 mg DPPH with a 100 ml of methanol and stored at 20°C until required. The working solution was bought by way of diluting DPPH solution with methanol. A 3 ml aliquot of this solution was blend with 100 μl of the specimen at various concentrations, starting from 10 to 500 μg/ml. The reaction mixture was shaken good, stored in the dark for 15 min at room temperature. An absorbance measured at 517 nm. The control was prepared by the same way, except any crude extract. The scavenging activity determined from the following equation:
% Scavenging = [ c o n t r o l a b s o r b a n c e − s a m p l e a b s o r b a n c e c o n t r o l a b s o r b a n c e ] × 100
Reducing power
The reducing power is basically the transformation of Fe (III) to Fe (II) in the presence of the crude extract or else (Fejes et al., 2000). The transformation to Fe (II) used to be monitored with the help of measuring the formation of Perl’s Prussian blue at 700 nm. More than a few concentrations of the sample (2 ml) had been blended with 2 ml of phosphate buffer (0.2 M, pH 6.6) and a 2 ml of potassium ferricyanide (10 mg/ml). The combination was incubated at 50°C for 20 min followed by the help of addition of 2 ml of trichloroacetic acid (100 mg/l). This mixture was centrifuged at 3000 rpm for 10 min, the supernatant of the solution was then removed carefully. A 2 ml solution from each and every of this mixture combined with 2 ml of distilled water and 0.4 ml of 0.1% (w/v) recently prepared ferric chloride. 10 min later, after reaction starts, the absorbance noted at 700 nm. A higher absorbance of the reaction mixture indicates a bigger reducing power capacity.
Total phenolic content measurement
The complete phenolic content was decided through the spectrophotometric method (Kim, 2003). 1 ml of sample solution (1 mg/ml) was mixed with 1 ml of Folin-Ciocalteu’s phenol reagent. Then 5 min later, 10 ml of a 7% Na2CO3 solution was introduced into that mixture adopted by means of the addition of 13 ml of deionized distilled water and mixed completely. The combination was allowed to stand for 90 min at 23°C and in the absence of light, after that the absorbance measured at 750 nm. The TPC was determined from extrapolation of calibration curve that have been extracted from gallic acid solution. The estimation of the phenolic compounds was implemented three times. The TPC was expressed as milligrams of gallic acid equivalents (GAE) per g of dried specimen.
Total flavonoid content
Total flavonoid content estimated according to the process described through(Park et al., 2008). A 0.3 ml of extracts dissolved with 3.4 ml of 30% methanol, 0.15 ml of NaNO2 (0.5 M) and 0.15 ml of AlCl3.6H2O (0.3M) in a 10 ml test tube. After 5 min, 1 ml of NaOH (1 M) was delivered to that combination. The blend was liberated well and the absorbance taken at 506 nm. The standard curve for whole flavonoids was made utilizing rutin solution (0 to 100 mg/l) under the similar process as earlier described. Total flavonoids have been expressed as milligrams of rutin equivalents per g of dried crude extract.
In vivo anti-inflammatory assay
Anti-inflammatory activity – Carrageenan induced paw edema
Animal selection
Healthy male rats weighing from 150-200 gm were selected for this study. They were kept at 12-hour dark and light cycle. Maintained with standard animal handling guidelines. The institutional ethical committee of Department of Pharmacy, International Islamic University, have been read and approved the use of rats on this protocol. The authentication number prescribed by the committee was Pharm-P&D-44/09’13-08.
Carrageenan
Carrageenan (lambda form, FMC Marine Colloids Division, NJ, or type IV, Sigma Aldrich, Poole, UK) was prepared as a 1% W/V solution in 0.9% saline, no more than 24-hour before use. The lambda form does not gel strongly at room temperature and is injectable to induce an inflammatory response. Anti-inflammation assay in inflamed rodent induced by carrageenan, was done according to the procedure originally described by Parasuraman et al., 2014.
Procedure
Animals had been weighed and randomized in 5 groups (n = 4). Before medication, the volume of the left paw of every animal was determined using a Plethysmometer. All of the animals were starved overnight prior to experiment, water used to be furnished ad libitum, to ensure uniform hydration. Group I served as control and did not receive any drug. Group II bought the normal Indomethacin (10 mg/kg, p.o) and Group III, IV, and V got crude extract in three different doses (100, 200, 300 mg/kg, p.o) accordingly. Thirty minutes following the subcutaneous injection of 0.1 ml of 1% w/v freshly ready λ-carrageenan solution into the planar facet of the left hind paw, every staff had acquired their prescribed medications. The paw was marked with ink on the stage of the lateral malleolus and immersed in the water reservoir of digital Plethysmometer as much as that mark to measure the paw volume. The paw volume (Vc) was measured at 2, 4, 6, 12 and 24 hour immediately after carrageenan injection in control and other treated groups (Vt) Azam et al., 2015Jimenez-Estrada et al., 2006. Percentage of inhibition of each group determined through the following formula:
% Inhibition = (Vc - Vt/ Vc) X 100
Statistical analysis
Information expressed here as mean ± SD from separate observations. For in vivo anti-inflammatory assays, one-way ANOVA scan adopted following Dunnet’s test (P < 0.05) correction. The values estimated from the Graph Pad Prism 6 application. The in vitro experiments had been analyzed by means of one-way ANOVA and each of them had been corrected by using Dunnet’s multiple comparison test with hypothetical value P < 0.05 as significant limit.
Results
Result of antioxidant activity
Figure 1 is showing the scavenging effects of crude sample on DPPH free radical. The EC50 value of scavenging of DPPH radicals by the methanolic crude extract were 196.47 ± 2.061 pg/ml and the reference drug, ascorbic acid had shown 41.31 ± 1.513 pg/ml of EC50 value ( Table 1 ). The study revealed that the antioxidant potential of the extract was found to be satisfactory (P < 0.05) in comparison of ascorbic acid.
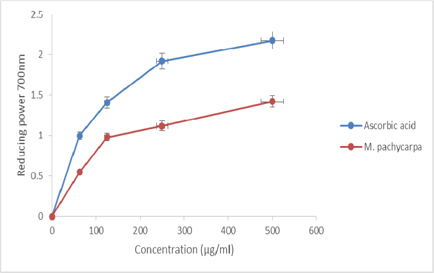
Figure 2 is representing the dose response curves for the reducing capability of methanolic extracts (62.5 - 500 μg/ml) from M. pachycarpa. It was discovered that the reducing power expanded with concentration proportionally. The major reduction has been perceived at 500 μg/ ml concentration (1.423 ± 0.057).
The methanolic extract of M. pachycarpa showed total phenolic contents of 41.23 ± 1.527 mg equivalent gallic acid per gram and provide a total flavonoid content of 110.33 ± 2.255 mg equivalent rutin per gram ( Table 2 ).
Result of in vivo anti-inflammatory activity
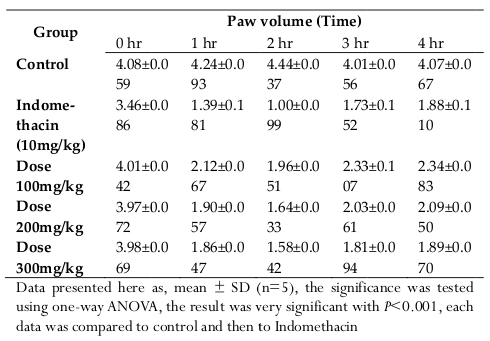
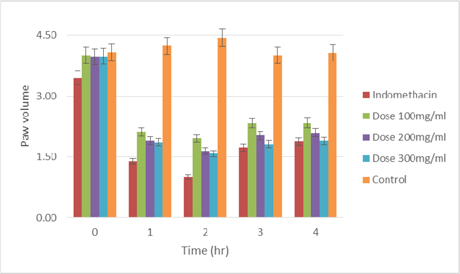
The extract were tested at three different doses (100,200 and 300 mg/kg), the extract exerted considerable inhibitory effect on paw swelling 1 hour after carrageenan administration, with more than 50% inhibition for all the three different dose ( Table 3 and Table 4 ). The maximum inhibition (61.36%, P < 0.001) elicited by the methanoliccrude extract was recorded at 2 hour, after carrageenan injection. Indomethacin, which was a reference drug, showed a similar inhibitory effect ( Figure 3 and Figure 4 ),at 2 hourof carrageenan administration it was able to exterminate paw edema by 75.47%.
Discussion
In vitro anti-oxidant study
A couple of methods had been utilized to determine the antioxidant property in vitro, as we all know components that have low antioxidative potency at in vitro, most by and large shows little response at in vivomodel Pereira et al., 2012. Free radicals are identified to liable for a broad variety of pathological conditions. Antioxidants help body immune system to oppose those free radicals and protect itself, they do that either by scavenging free radicals or defending the defense mechanism Umamaheswari and Chatterjee, 2008. The DPPH free radical assay is well reputed for evaluating electron donation potential of average crude extracts, where a purple-colored solution bleaching acts like an indicator Pereira et al., 2012. This approach based on scavenging, DPPH radicals are engulfed via the antioxidant factor that decolorizes the DPPH solution. The extent of color change is directly proportional to the concentration and potency of the antioxidants. A significant decrease in the absorbance of the reaction indicates the presence of a large quantity of valuable free-radical scavenging compounds within the experimental specimen Krishnaiah et al., 2011. In our present standing with methanolic extract of M. pachycarpa, it has confirmed tremendously bigger percentage of inhibition. As a result, from this study we can make a proposal, that the plant extract contain distinctive phytochemical components which are able of donating hydrogen to a free radical to lessen, either complete or partial, the vulnerability of injury.
In the assay of reducing power capacity, the yellow color of the checks response mixture changes to green and it will depend on the reducing capability of the test extract. If there are any reductant persist within the specimen, it will shrink the Fe3+ /ferricyanide complexion to its ferrous type. For this reason, Fe2+ is measured through absorbance studying at 700 nm. Prior studies suggesting, that the reducing movements exerted through antioxidant action are due to the donation of a hydrogen atom to break the free radical chain (Gordon, 1990). The greater the absorbance at 700 nm, the increased reduction potentiality Saeed et al.,2012.
Phenolic compounds are viewed as secondary metabolites, they are derived from phenylalanine, and tyrosine occurs naturally in plants and are differentiated Naczk and Shahidi, 2004. The methanol crude extract has been exerted an excellent total phenolic contents, despite the fact that this contents got previous reviews, were so much smaller Ao et al., 2008. Phenolic compounds are additionally most important for antioxidant property, seeing that its sensible staff (OH-) liberate the scavenging capability. Phenolic compounds are categorized in a few class; amongst them flavonoids are regarded as a most robust antioxidant. Flavonoids naturally occur in herbs and they have constructive results on human well-being. Past acknowledgement on flavonoid derivatives, states that it plays a great function in the efficiency of distinct plants antibacterial, antiviral, anti-inflammatory, anticancer, and anti-allergic activities Di Carlo et al., 1999Montoro et al., 2005. Therefore, in comparison with the other findings from the different extracts of plant extracts Sahreen et al., 2011, our present observation recommend that, the contribution of phenolic acids and flavonoids could also play the fundamental role in the antioxidant action. Due to the fact that the EC50values of the radical scavenging action of methanolic extract of M. pachycarpa are radically high and the phenolic and flavonoid content material was high, as good. Nevertheless, one of a kind phenolic compound have an exceptional extent of responses toward the Folin-Ciocalteu method. In a similar way, the molecular antioxidant response of phenolic compounds varies remarkably, depending on their chemical constitution; alternatively, the effect may be interfered by means of different chemicals presence within the extract, such as sugars Saeed et al., 2012Satue-Gracia et al., 1997Singleton and Rossi, 1965.
In vivo anti-inflammatory activity
Carrageenan brought on paw edema is an approach that may be characterized by using biphasic occasion with the involvement of various inflammatory mediators. The primary section starts with (within first 2 hours after carrageenan injection) chemical mediators corresponding to histamine and serotonin, wherein second phase (after 2 hours of carrageenan injection) Kinins and prostaglandins performs their position in the infection procedure Azam et al., 2015. Our outcome published that administration of M. pachycarpa extract inhibits irritation, which was brought on by way of Carrageenan. Prior study on this plant extract also suggest that it has an effective molecule in its phytochemistry that may avoid inflammation by protecting cell lysis Chowdhury A, 2013.
It has been said that presence of certain flavonoids exerts profound anti-inflammatory activity by stabilizing the lysosomal membrane Oyedapo et al., 2010Read, 1995. The outcome of our study of and from the previous database on this plant, it can be predictable that the anti-inflammatory effect exerted is because of flavonoid content. The anti-inflammatory activity of M. pachycarpa extracts via edema inhibition indicates a lot-massive effect in comparison with Indomethacin. Carrageenan-induced paw edema model is a suitable experimental animal model for evaluating or screening the anti-inflammatory effects from natural products.
Sometimes it happens that the crude plant extracts are extra pharmacologically lively than their isolated active compounds Hamburger and Hostettmann, 1991. The targeted mechanism of action for the antiinflammatory activity of studied specimen just is not identified, but the extract may intercepting the construction of inflammatory mediators dependable for inflammation, either COX pathway or different specific enzymatic mechanism.
Conclusion
In the present investigation, we have revealed that the methanolic extract of M. pachycarpa has amazing antioxidant and anti-inflammatory properties, which supports the usual use of this plant as well as its prior findings. The replacement of synthetic with traditional antioxidants (due to human safety issue) is superb. Our study showed good free radical scavenging endeavor and persistence of total phenolic and flavonoid content. The anti-inflammatory activity exerted with the aid of this extract also thought to be correlated with its anti-oxidant property. As a consequence, this plant extracts deserves to get higher priority when a new molecule is being searched in that certain object.
References
-
C.
Ao,
A.
Li,
A.A.
Elzaawely,
T.D.
Xuan,
S.
Tawata.
Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control.
2008;
19
:
940-948
.
-
O.I.
Aruoma.
Free radicals, oxidative stress, and antioxidants in human health and disease. Journal of the American Oil Chemists' Society.
1998;
75
:
199-212
.
-
S.
Azam,
A.F.
Huda,
K.
Shams,
P.
Ansari,
M.K.
Mohamed,
M.M.
Hasan,
A.K.
Azad,
K.K.
Mondal,
S.M.
Zaouad.
Anti-Inflammatory and Anti-Oxidant Study of Ethanolic Extract of Mimosa pudica. Journal of Young Pharmacists.
2015;
7
:
234-240
.
-
W.
Brand-Williams,
M.E.
Cuvelier,
C.
Berset.
Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology.
1995;
28
:
25-30
.
-
E.
Bursal,
İ.
Gülçin.
Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Research International.
2011;
44
:
1482-1489
.
-
M.A.
Chowdhury A,
Azam S
Rahman S.
Human red blood cell membrane stability testing for the estimation of anti-inflammatory activity of methanolic extract of Millettia pachycarpa Benth leaves. IntJPharma Sci Res.
2013;
4(12)
:
4587-4590
.
-
R.H.
Delaporte,
G.M.n.
Sánchez,
A.C.
Cuellar,
A.
Giuliani,
J.C.
Palazzo de Mello.
Anti-inflammatory activity and lipid peroxidation inhibition of iridoid lamiide isolated from Bouchea fluminensis (Vell.) Mold. (Verbenaceae). Journal of Ethnopharmacology.
2002;
82
:
127-130
.
-
G.
Di Carlo,
N.
Mascolo,
A.A.
Izzo,
F.
Capasso.
Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sciences.
1999;
65
:
337-353
.
-
C.A.
Dinarello.
Anti-inflammatory Agents: Present and Future. Cell.
2010;
140
:
935-950
.
-
A.
Djeridane,
M.
Yousfi,
B.
Nadjemi,
D.
Boutassouna,
P.
Stocker,
N.
Vidal.
Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chemistry.
2006;
97
:
654660
.
-
S.
Fejes,
A.
Blázovics,
A.
Lugasi,
É.
Lemberkovics,
G.
Petri,
Á.
Kéry.
In vitro antioxidant activity of Anthriscus cerefolium L. (Hoffm.) extracts. Journal of Ethnopharmacology.
2000;
69
:
259-265
.
-
A.
Geronikaki,
A.
Gavalas.
Antioxidants and Inflammatory Disease: Synthetic and Natural Antioxidants with Anti-Inflammatory Activity. Combinatorial Chemistry & High Throughput Screening.
2006;
9
:
425-442
.
-
H.
Göçer,
İ.
Gülçin.
Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. International Journal of Food Sciences and Nutrition.
2011;
62
:
821-825
.
-
M.H.
Gordon.
The Mechanism of Antioxidant Action in Vitro. In Food Antioxidants (Springer Science + Business Media).
1990;
:
1-18
.
-
İ.
Gülçin.
Antioxidant activity of food constituents: an overview. Archives of Toxicology.
2011;
86
:
345-391
.
-
İ.
Gülçin,
E.
Bursal,
M.H.
Şehitoğlu,
M.
Bilsel,
A.C.
Gören.
Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food and Chemical Toxicology.
2010;
48
:
2227-2238
.
-
Z.
H.
Observation on curative effect of Huteng Tang (Huzhang and Millettia Combination) in treating side effects caused by cancer chemotherapy. Practical JInteg Chinese Western Med.
1996;
9(3)
:
137
.
-
M.
Hamburger,
K.
Hostettmann.
7. Bioactivity in plants: the link between phytochemistry and medicine. Phytochemistry.
1991;
30
:
3864-3874
.
-
M.
Jainul.
Evaluation of Thrombolytic Effect of Seven Different Bangladeshi Plants. BJPR.
2014;
4
:
1400-1406
.
-
A.S.
Jainul MA,
A
Chowdhury.
In vitro cytotoxic activity of methanolic extract of M. pachycarpa (Benth) leaves. The Pharm Innova.
2013;
2(1)
:
10-13
.
-
M.
Jimenez-Estrada,
R.R.
Chilpa,
T.R.
Apan,
F.
Lledias,
W.
Hansberg,
D.
Arrieta,
F.J.A.
Aguilar.
Anti-inflammatory activity of cacalol and cacalone sesquiterpenes isolated from Psacalium decompositum. Journal of Ethnopharmacology.
2006;
105
:
34-38
.
-
L.
K.
Mosquitocidal activity of Millettia pachycarpa on the larvae and eggs of Aedis aegypti. Annal Biol Res.
2011;
2(3)
:
217-222
.
-
D.
Kim.
Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry.
2003;
81
:
321-326
.
-
P.M.
Kris-Etherton,
M.
Lefevre,
G.R.
Beecher,
M.D.
Gross,
C.L.
Keen,
T.D.
Etherton.
BIOACTIVE COMPOUNDS IN NUTRITION AND HEALTH-RESEARCH METHODOLOGIES FOR ESTABLISHING BIOLOGICAL FUNCTION: The Antioxidant and Antiinflammatory Effects of Flavonoids on Atherosclerosis. Annu Rev Nutr.
2004;
24
:
511-538
.
-
D.
Krishnaiah,
R.
Sarbatly,
R.
Nithyanandam.
A review of the antioxidant potential of medicinal plant species. Food and Bioproducts Processing.
2011;
89
:
217-233
.
-
P.
Montoro,
A.
Braca,
C.
Pizza,
N.
Detommasi.
Structure?antioxidant activity relationships of flavonoids isolated from different plant species. Food Chemistry.
2005;
92
:
349-355
.
-
M.
Naczk,
F.
Shahidi.
Extraction and analysis of phenolics in food. Journal of Chromatography A.
2004;
1054
:
95-111
.
-
K.A.
Oluyemi,
U.C.
Okwuonu,
D.G.
Baxter,
T.O.
Oyesola.
Toxic Effects of Methanolic Extract of Aspilia africana Leaf on the Estrous Cycle and Uterine Tissues of Wistar Rats. Int J Morphol.
2007;
25
.
-
O.
Oyedapo,
B.
Akinpelu,
K.
Akinwunmi,
M.
Adeyinka,
F.
Sipeolu.
Red blood cell membrane stabilizing potentials of extracts of Lantana camara and its fractions. Int J Plant Physiol Biochem.
2010;
2
:
46-51
.
-
O.A.
Oyedapo,
C.O.
Adewunmi,
E.O.
Iwalewa,
V.O.
Makanju.
Analgesic, Antioxidant and Anti-inflammatory Related Activities of 21-hydroxy-2,41-dimethoxychalcone and 4-hydroxychalcone in Mice. J of Biological Sciences.
2007;
8
:
131-136
.
-
N.
Ozsoy,
E.
Candoken,
N.
Akev.
Implications for Degenerative Disorders: Antioxidative Activity, Total Phenols, Flavonoids, Ascorbic Acid, ß-Carotene and ß-Tocopherol in Aloe vera. Oxidative Medicine and Cellular Longevity.
2009;
2
:
99-106
.
-
S.
Parasuraman,
R.
Petchi,
C.
Vijaya,
S.
Dhanaraj.
Evaluation of free radical scavenging properties and hypoglycemic activityof ethanolic extract of Tridax procumbens Linn. in Wistar rats. Drug Dev Ther.
2014;
5
:
164
.
-
Y.-S.
Park,
S.-T.
Jung,
S.-G.
Kang,
B.G.
Heo,
P.
Arancibia-Avila,
F.
Toledo,
J.
Drzewiecki,
J.
Namiesnik,
S.
Gorinstein.
Antioxidants and proteins in ethylene-treated kiwifruits. Food Chemistry.
2008;
107
:
640-648
.
-
X.
Pereira,
F.
Souza,
J.R.G.
da S. Almeida,
J.T.
de Lima,
L.A.d.
Arajo Ribeiro,
L.J.
Quintans Jnior,
J.M.
Barbosa Filho.
Biological Oxidations and Antioxidant Activity of Natural Products. In Phytochemicals as Nutraceuticals - Global Approaches to Their Role in Nutrition and Health (InTech).
2012
.
-
S.B.
Racette,
X.
Lin,
M.
Lefevre,
C.A.
Spearie,
M.M.
Most,
L.
Ma,
R.E.
Ostlund.
Dose effects of dietary phytosterols on cholesterol metabolism: a controlled feeding study. American Journal of Clinical Nutrition.
2009;
91
:
32-38
.
-
S.
RC.
Traditional knowledge of Nyishi (Dafla) tribe of Arunachal Pradesh. Indian J Trad Know.
2010;
9(1)
:
26-37
.
-
M.A.
Read.
Flavonoids: naturally occurring anti-inflammatory agents. The American journal of pathology.
1995;
147
:
235
.
-
N.
Saeed,
M.R.
Khan,
M.
Shabbir.
Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complementary and Alternative Medicine.
2012;
12
:
221
.
-
S.
Sahreen,
M.R.
Khan,
R.A.
Khan.
Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. Leaves. J Med Plants Res.
2011;
5
:
2755-2765
.
-
J.A.
Sakat SS,
MN
Gambhire.
In vitro anti-oxidant and antiinflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharma Sci.
2010;
2(1)
:
146-155
.
-
N.B.
Samad,
T.
Debnath,
H.L.
Jin,
B.R.
Lee,
P.J.
Park,
S.Y.
Lee,
B.O.
Lim.
Antioxidant activity of Benincasa hispida seeds. Journal of Food Biochemistry.
2013;
37
:
388-395
.
-
M.
Satue-Gracia,
M.
Heinonen,
E.
Frankel.
Antioxidant activity of anthocyanin in LDL and lecithin liposome systems. Journal of Agricultural and Food Chemistry.
1997;
45
:
3362-3367
.
-
V.
Singleton,
J.A.
Rossi.
Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture.
1965;
16
:
144-158
.
-
F.
Tattersfield,
J.T.
Martin,
F.N.
Howes.
Some Fish-Poison Plants and Their Insecticidal Properties. Bulletin of Miscellaneous Information (Royal Gardens, Kew) 1940.
1940;
:
169
.
-
M.
Umamaheswari,
T.K.
Chatterjee.
In vitro antioxidant activities of the fractions of Coccinia grandis l. leaf extract. African Journal of Traditional, Complementary and Alternative Medicines.
2008;
5
.
-
H.O.
Vongtau,
J.
Abbah,
B.A.
Chindo,
O.
Mosugu,
A.O.
Salawu,
H.O.
Kwanashie,
K.S.
Gamaniel.
Central Inhibitory Effects of the Methanol Extract of Neorautanenia mitis. Root in Rats and Mice. Pharmaceutical Biology.
2005;
43
:
113-120
.
-
M.B.
Wannes WA,
Jemia MB
Sriti J.
Antioxidant activities of the essential oil and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem Toxicol.
2010;
48
:
1362-1370
.
-
T.
Wu,
J.
Fu,
Y.
Yang,
L.
Zhang,
J.
Han.
The effects of phytosterols/stanols on blood lipid profiles: a systematic review with meta-analysis. Asia Pac J Clin Nutr.
2009;
18
:
179-186
.
-
C.Y.
Zengin G,
Aktumsek A
Guler GO.
Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Rec Nat Prod.
2011;
5
:
123-132
.
Comments

Downloads
Article Details
Volume & Issue : Vol 2 No 10 (2015)
Page No.: 366-373
Published on: 2015-10-05
Citations
Copyrights & License
Search Panel
- HTML viewed - 8675 times
- Download PDF downloaded - 2078 times
- View Article downloaded - 7 times
- Test PMC downloaded - 6 times
 Biomedpress
Biomedpress