Abstract
Methods: The L. cubeba fruit hydrosol was obtained by steam distillation method. Evaluation of the growth-inhibiting and microbicidal effects of the hydrosol towards the H. pylori ATCC 43504 and C. albicans ATCC 10231 was determined through MIC (minimal inhibitory concentration), MBC (minimal bactericidal concentration), and MFC (minimal fungicidal concentration) measurements using broth dilution assays. Compositions of the dissolved essential oil (dEO) from the hydrosol were analyzed by GC-MS (gas chromatography-mass spectrometry).
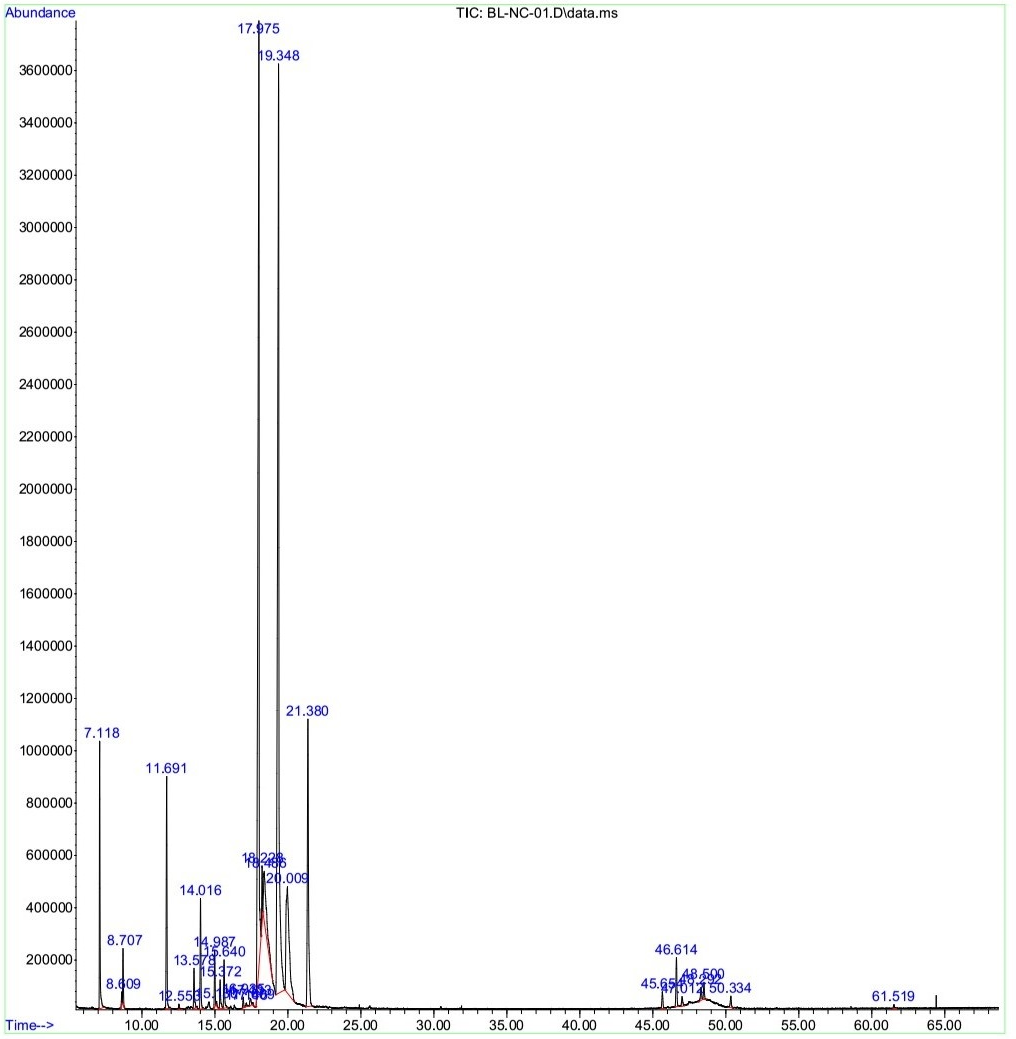
Results: The results indicated that the L. cubeba fruit hydrosol exhibited strong antimicrobial ability towards the bacterium H. pylori (MIC of 10%, MBC of 30%) and the yeast C. albicans (MIC of 10%, MFC of 40%). The cells of H. pylori and C. albicans were killed completely after 24 and 18 hours of treatment with 30% and 40% of the hydrosol, respectively. The major constituents of the dEO were geranial (32.92%), neral (27.12%), p-menthan-8-yl acetate (8.45%), 2-cyclopropyl-2-methylspiro[2.2]pentane-1-carboxylic acid (8.09%), linalool (4.24%), and methyl heptenone (4.15%).
Conclusion: The results of the study suggest that L. cubeba fruit hydrosols could be used as potent natural antibacterial and antifungal preparations in the global effort to discover safe alternatives to toxic antimicrobial agents.
Introduction
Hydrosol is an aqueous layer (or aromatic water) located just below the essential oil (EO) layer during steam distillation of aromatic and medicinal plant materials. Hydrosol is the byproduct of steam distillation of EOs. Almost all hydrosols contain small quantities of dissolved essential oil (dEO). However, some plants contain a high amount of dEO1. Since hydrosols possess a wide range of beneficial properties, such as pH 5.5, pleasant scents, relaxing fragrance and antimicrobial ability, they are traditionally used in cosmetics, food applications, and medicinal purposes in many countries around the world2, 3. These days, hydrosols have attracted an increasing number of researchers because of their eco-friendly, health-benefitting and safe characteristics2, 3. Several recent studies have demonstrated that hydrosols from different plants exhibited strong antimicrobial activities against a wide variety of fungi and bacteria2, 4, 5, 6, 7. Therefore, research on hydrosols would bring promising solutions for controlling pathogenic fungi and bacteria.
Helicobacter pylori and Candida albicans are two of the most common human pathogens that cause a wide range of serious diseases for humans. H. pylori is a Gram-negative spiral bacterium with the ability to produce urease, persistently colonizing in the gastric mucosa of at least half of the world’s population8, 9. In some developing countries, the rate of infection with H. pylori can reach up to 80% or higher10. The bacterium has been proven to be responsible for gastroduodenal diseases and is greatly associated with gastric carcinogenesis 11, 12. C. albicans is a polymorphic fungus that is the common infectious agent of the skin, oral cavity and esophagus, gastrointestinal tract, vagina, and vascular system of humans 13, 14. The fungus, which is uniquely adapted to its human host, often exists as a harmless organism at various mucosa15, 16. However, the pathogen can cause serious conditions under certain circumstances, including life-threatening systemic infection14.
Antibiotic and antifungal therapies were successful in controlling the H. pylori and C. albicans infections, greatly contributing to reduction of diseases caused by these pathogens 17, 18. However, the side effects which resulted from the drugs and the rapid emergence of drug-resistant strains have made the therapies for the treatment of H. pylori and C. albicans lose their effectiveness 19, 20, 21. Hence, there is an urgent need for new antimicrobial agents for more effective management of these infectious agents.
Increasing public concern about healthy and natural products nowadays has promoted much research focusing on developing new medicines from plant sources. Numerous extracts and EOs were demonstrated to exhibit strong antimicrobial activities towards H. pylori and C. albicans without any resistance development of these microbes 22, 23, 24, 25, 26. Fruit of Litsea cubeba, a member of Lauraceae family, has been used for stimulating the digestive system and in treatment of stomach ache in Vietnam 27, 28, 29 and Malaysia 30. The EO from the fruit of Litsea cubeba was found to have a strong activity against both Gram-negative and Gram-positive bacteria 31, 32, 33. In addition, the EO and terpenoid extraction from fruits of L. cubeba exhibited effective activity against several pathogenic fungi in plants and humans, including C. albicans34, 35. However, no information has been obtained related to the constituents and the potential of the hydrosol from the L. cubeba fruit to control H. pylori and C. albicans.
In the present study, the growth-inhibiting, bactericidal, and fungicidal effects of the hydrosol extracted from steam distillation of the L. cubeba fruit against H. pylori ATCC 43504 and C. albicans ATCC 10231 were assessed. Chemical constituents of dEO extracted from the hydrosol were also determined by gas chromatography-mass spectrometry (GC-MS).
Materials and Methods
Plant collection
The fresh samples of Litsea cubeba (Lour.) Pers. (1806) were collected from the Mang Den Medicinal Plants Garden, in Kon Tum province (the Central Highlands of Vietnam) in June 2018. The identification to species of the specimen was conducted by the method of morphological descriptions. A voucher specimen has been deposited in the PHH Herbarium of Vietnam National University Ho Chi Minh City.
Preparation of hydrosol
An amount of 800 gram of fresh fruit of L. cubeba was finely ground in a mixer and then subjected to steam distillation at 100°C using a Clevenger-type apparatus with 3000 mL of water for 3 hours. The hydrosol was separated by a separation funnel; afterwards, the hydrosol (~1800 mL) was obtained and stored in dark bottles at 4°C until used.
Reagents
Brucella broth (BB), brain heart infusion broth (BHIB), sabouraud dextrose agar (SDA), and sabouraud dextrose broth (SDB) were purchased from Becton Dickinson, Inc. (Sparks, MD, USA). Newborn bovine serum (NBS) was obtained from Hyclone (Longan, UT, USA). Amoxicillin (≥98%) was purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Nystatin was provided by Merck (Kenilworth, NJ, USA). All other chemicals and reagents used in this study were of analytical grade quality and available commercially.
Microbial strains and culture conditions
The reference strains of H. pylori ATCC 43504 and C. albicans ATCC 10231 were provided by the Oxford University Clinical Research Unit Vietnam (OUCRU - VN) and Department of Plant Biotechnology and Biotransformation, Faculty of Biology and Biotechnology, University of Science, VNU - HCM, respectively. Authentication of these strains was done using MALDI-TOF MS (OUCRU - VN). The bacterial and the yeast strains were stored in BHIB and SDB media, respectively, supplemented with 25% glycerol and placed in a nitrogen liquid container until use.
The H. pylori strain was cultured on Brucella agar added with 10% NBS, and then incubated at 37oC for 3 days under a microaerophilic condition created by a 2.5 L Oxoid AnaeroJar and Oxoid CampyGen sachet (Thermo Fisher Scientific, Waltham, MA, USA). The bacterial suspensions (~ 5x107 CFU/mL) used for the bioassay were made in Brucella broth using a 72-h subculture of H. pylori on Brucella agar. The C. albicans strain was activated in SDB medium at 37oC for 2 days. The yeast suspensions for bioassay (~ 5x106 CFU/mL) were prepared in SDB medium from a 48-h subculture of the activated C. albicans. Microbial density was determined using McFarland turbidity standards.
Separation and analysis of chemical constituents of dissolved essential oil
Dissolved essential oil (dEO) was extracted by vigorously shaking the hydrosol (900 mL) with hexane (10:1, v/v) for 30 minutes using the separation funnel. The mixture was allowed to settle and the hexane layer saturated with the dEO was separated from the water layer. The hexane layer was then evaporated at 42oC under reduced pressure to obtain the dEO. The remaining water was removed from dEO by using anhydrous sodium sulphate. The yield of the dEO was determined based on the fresh weight of the sample. The dEO was kept in a dark vial and put in a fridge at -20°C until analyzed.
The dEO was analyzed by GC-MS, conducted at the Central Lab for Analysis, University of Science, VNU - HCM. In brief, 1 µL of the dEO was diluted in 1 mL hexane, and then 1 µL of the solution was analyzed by GC-MS using a Agilent 6890N gas chromatograph coupled to a mass spectrometer (Agilent 5975C inert MSD, Santa Clara, CA, USA). Compositions were separated on a fused silica capillary column (HP-5MS) coated with polydimethylsiloxane (60 m × 0.32 mm internal diameter, 0.30 μm film thickness). The following oven temperature program was initiated at 50°C for 2 minutes, ramped at a rate of 2°C/minute to 80°C, 5°C/minute to 150°C, 10°C/minute to 200°C, and then increased at the rate of 20°C/minute to a final temperature of 300°C and held for 5 minutes. The MS operating parameters were an ionization voltage of 70 eV and electron multiplier energy of 1,024 V. Injector, interface, and ion source were kept at 220, 250 and 230°C, respectively. Compound identifications were based on comparisons of their mass spectra (MS) with the MS obtained from a MS database of the National Institute for Standard Technology (NIST) (USA/Wiley, 2011).
Microbiological Assay
Broth dilution assay
The values of MICs (Minimal Inhibitory Concentrations) and MBCs/MFCs (Minimal Bactericidal/Fungicidal Concentrations) were determined by the method of broth dilution in sterile 15 mL test tubes 26, 36. In brief, an amount of 100 mL bacterial suspension (~ 5x107 CFU/mL) or yeast suspension (~ 5x106 CFU/mL) was dispensed into each tube containing 1.9 mL of culture medium (BB medium for H. pylori and SDB medium for C. albicans)- without or with the hydrosol at various percentages of 10, 20, 30, 40, or 50% (v/v). The tubes were then shaken at 150 rpm and incubated at 37°C for 48 hours. After incubation, 50 μL of resazurin indicator solution (0.01%) was added to each test tube. After the post incubation for 1 hour at 37°C, the change of color in each tube was assessed visually. MIC values of the hydrosol against the microbial strains were determined as the lowest percentage values at which blue color of the indicator remained (indicating no microorganism growth) or changed from blue to slightly purple (equivalent to prominent growth inhibition). Test tubes containing bacterial or yeast suspensions, culture medium, and sterile distilled water were used as negative controls. Antibiotic amoxicillin and antifungal nystatin (as positive controls) were used to assure the reliability of the experiment results, and were similarly prepared. All bioassays were repeated three independent times and on triplicate samples.
In order to determine the MBC/MFC values, the suspensions of the tubes without the color change of resazurin in the MIC assay were taken and made into 10-fold serial dilutions. Subsequently, the dilutions (each 100 μL) were spread on the surface of Petri dishes with Brucella agar and SDA for H. pylori and C. albicans, respectively. The dishes were incubated at 37oC for 3 days, and the growth of the microorganisms was then checked. The lowest hydrosol percentage values that showed no growth on the subcultures were determined as MBC or MFC values.
Time-killing assay
The growth curves of H. pylori ATCC 43504 and C. albicans ATCC 10231 treated with the hydrosol 10, 20, 30 and 40% (v/v) were established. An amount of 100 μL of the microbial suspensions was inoculated onto test tubes containing 1.9 mL of cuture medium alone (control curve) or containing 10, 20, 30 or 40% of the hydrosol. Then, the test tubes were put in the incubator at 37oC with shaking (150 rpm) for 0, 6, 12, 18, 24, 36, and 48 hours of incubation. At each of the incubation times, the suspensions were taken and 10-fold serial dilutions were made. The dilutions (each 100 μL) were spread on the surface of Petri dishes with Brucella agar and SDA for H. pylori and C. albicans, respectively. After the 3-day incubation, colonies were counted by a plate colony count technique.
Data analysis
All bioassays were repeated three to five times in triplicate, and mean values ± SD were presented. The Bonferroni multiple-comparison method was used to test for significant differences among the treatments using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Chemical constituents of the dissolved essential oil from the L. cubeba fruit hydrosol
The dissolved essential oil (dEO) extracted from L. cubeba fruit hydrosol had a yield of 0.18% (w/w on fresh weight basis). The constituents of the dEO were identified by GC-MS and presented in Table 1. The results showed that the main components of the dEO were as follows: geranial (32.92%), neral (27.12%), p-menthan-8-yl acetate (8.45%), 2-cyclopropyl-2-methylspiro[2.2]pentane-1-carboxylic acid (8.09%), linalool (4.24%), methyl heptenone (4.15%), (R)-(+)-citronellal (2,10%), α-terpineol (1.04%), and terpinen-4-ol (1.02%), which accounted for 89.13% and 0.1604% of the total dEO content and the hydrosol, respectively (Figure 1). The minor and unidentified compounds of the dEO accounted for 2.76% and 8.11%, respectively. Out of 15 determined compounds, there were 14 oxygenated components (91.24%) and one hydrocarbon only (0.65%) (Table 1).
| No. | Chemical Compound | Molecular formula | Retention time (minute) | dEO (%) | Hydrosol (%) |
|---|---|---|---|---|---|
| 1 | Methyl heptenone | C8H14O | 7.118 | 4.15 | 0.0075 |
| 2 | 3,4-pentadienal | C5H6O | 8.609 | 0.20 | 0.0004 |
| 3 | Eucalyptol | C10H18O | 8.707 | 0.85 | 0.0015 |
| 4 | Linalool | C10H18O | 11.691 | 4.24 | 0.0076 |
| 5 | Octanol acetate | C10H20O2 | 12.553 | 0.15 | 0.0003 |
| 6 | Isopulegol | C10H18O | 13.578 | 0.82 | 0.0015 |
| 7 | (R)-(+)-citronellal | C10H18O | 14.016 | 2.10 | 0.0038 |
| 8 | Terpinen-4-ol | C10H18O | 14.987 | 1.02 | 0.0018 |
| 9 | Santolinyl acetate | C12H20O2 | 15.127 | 0.09 | 0.0002 |
| 10 | 2,5-octadiene | C8H14 | 15.372 | 0.65 | 0.0012 |
| 11 | α-terpineol | C10H18O | 15.640 | 1.04 | 0.0019 |
| 12 | Neral | C10H16O | 17.975 | 27.12 | 0.0488 |
| 13 | Geranial | C10H16O | 19.348 | 32.92 | 0.0593 |
| 14 | 2-cyclopropyl-2-methylspiro[2.2]pentane-1-carboxylic acid | C12H14O2 | 20.009 | 8.09 | 0.0146 |
| 15 | -menthan-8-yl acetate | C12H22O2 | 21.380 | 8.45 | 0.0152 |
| Total | 91.89 | 0.1654 | |||
Antimicrobial effects of the L. cubeba fruit hydrosol on H. pylori and C. albicans
The MIC and MBC values of the hydrosol towards the H. pylori strain ATCC 43504 are shown in Table 2. The hydrosol displayed a high growth inhibitory activity with a MIC value of 10% against the H. pylori strain. The hydrosol also exhibited a relatively strong bactericidal activity towards the bacterial strain with an MBC value of 30%.
| Organism | Sample | MIC | MBC/MFC |
|---|---|---|---|
| H. pylori strain ATCC 43504 | Hydrosol | 10 % | 30 % |
| Amoxicillin | 0.04 μg/mL | 0.10 μg/mL | |
| C. albicans strain ATCC 10231 | Hydrosol | 10 % | 40 % |
| Nystatin | 4 μg/mL | 16 μg/mL |
The growth-inhibiting and fungicidal effects of the hydrosol on the C. albicans strain ATCC 10231 are shown in Table 1. The hydrosol inhibited the growth of the yeast with the same MIC value (10%), in comparison with its effect on H. pylori. These results also indicate that the hydrosol had a potential fungicidal activity on the C. albicans strain with an MFC value of 40%.
Microbicidal time-kill of the L. cubeba fruit hydrosol against H. pylori and C. albicans
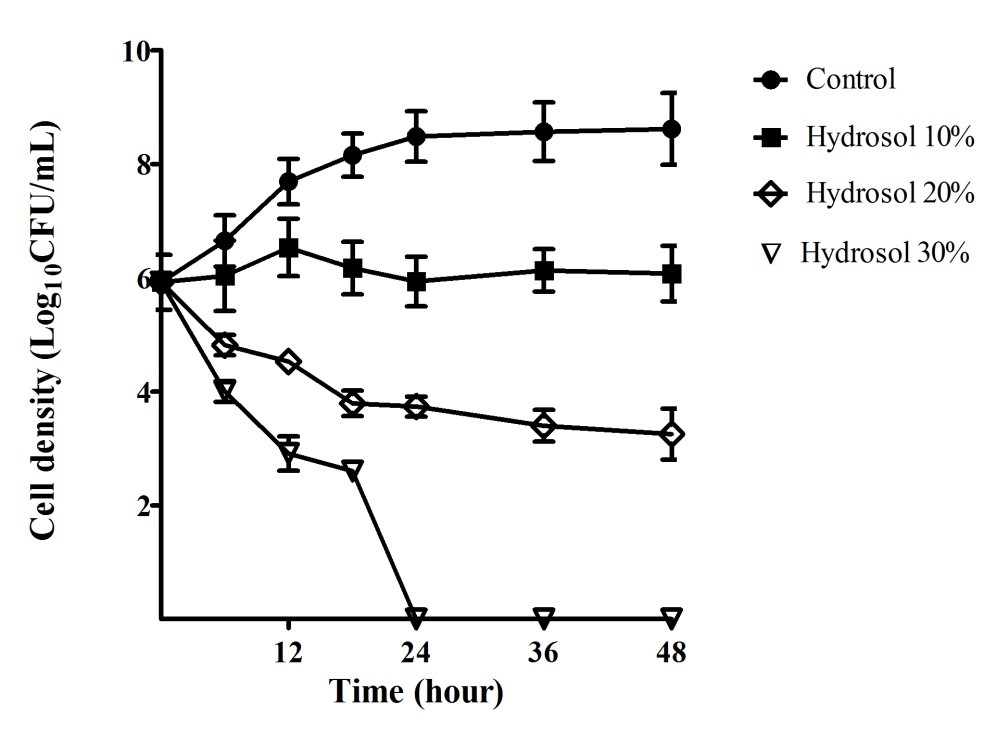
The growth curves of H. pylori ATCC 43504 were performed in order to evaluate the H. pylori bactericidal activity of the hydrosol over time. The results provided in Figure 2 indicated that the bacterial cells grew in a log phase from the start to 18 hours of the culture (5.92 – 8.15 Log10CFU/mL) and in a stationary phase (8.48 – 8.62 Log10CFU/mL) after 18 – 48 hours in the negative control culture. In the cultures with the hydrosol, the viable count of the bacteria decreased in a concentration- and time-dependent manner. The H. pylori population treated with 10%, 20% and 30% of the hydrosol decreased significantly (p<0.05) compared with the negative control after 12 - 48 hours. In the cultures treated with 10% hydrosol, the H. pylori strain was inhibited and could survive for 48 hours with no significant change in cell density (5.92 – 6.08 Log10CFU/mL). The populations of the test strains declined significantly in the treatments of H. pylori with the hydrosol (20%) with cell density ranging from 3.74 – 3.25 Log10CFU/mL in comparison with 8.48 – 8.62 Log10CFU/mL for the controls after 24 – 48 hours of treatment. In the cultures of 30% hydrosol, the microbial strains had sharp decreases in the viable count- from 5.92 to 2.61 Log10CFU/mL after 18 hours- and completely killed after 24 hours of treatment.
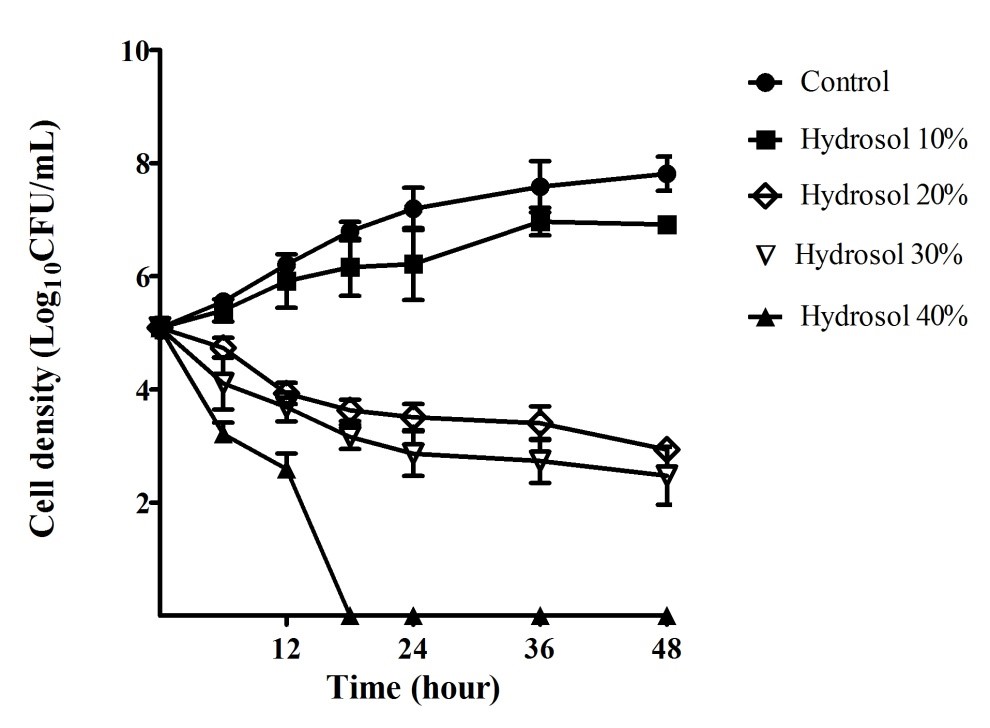
Fungal time-kill curves of C. albicans after exposure to the hydrosol at various percentages were also determined and are shown in Figure 3. In the negative control cultures, the yeast population increased steadily (5.09 – 7.81 Log10CFU/mL) during the period of 48 hours. In the cultures treated with the hydrosol, the growth of the yeast populations reduced in a concentration- and time-dependent manner. The C. albicans population gradually increased (5.09 – 6.92 Log10CFU/mL) for 48 hours treatment with 10% of hydrosol, but the growth was significantly slower (p<0.05) than that in the control culture at 48 h (7.81 Log10CFU/mL). The C. albicans population treated with 20%, 30% and 40% of the hydrosol decreased significantly (p<0.05), compared with the negative control after 12 - 48 hours. When treated with the hydrosol 20% and 30%, the number of the yeast cells had a significant fall (p<0.05) after 24 – 48 hours of treatments with cell density ranging 3.51 – 2.94 and 2.86 – 2.48 Log10CFU/mL, in comparison with 7.19 – 7.81 Log10CFU/mL of the controls, respectively. The exposure to the hydrosol 40% caused the yeast population to obviously drop, ranging from 5.09 to 2.60 Log10CFU/mL after 12 hours and completely killed after 18 hours of treatment.
Discussion
Hydrosols from some plants were identified chemical constituents 37, 38, 39, 40, 41, but there was no information about the composition of the L. cubeba fruit hydrosol. The previous studies of EOs from L. cubeba fruits collected in Vietnam, China, and India indicated that neral and geranial were the two main components of the EOs (66.3 – 83.9%) 42, 43, 44. Both of the two compounds were also the predominant constituents accounting for 60.04% of the dEO from the hydrosol in our study. Furthermore, other compounds, including methyl heptenone, eucalyptol, linalool, isopulegol, citronellal, terpinen-4-ol, and α-terpineol, were found present in the fruit EOs in several previous studies 42, 43, 44. Interestingly, they were also found in the hydrosol in our study herein. In addition, the dEO in the hydrosol had hydrophilic properties because it mainly contained oxygenated compounds (91.24%) yet with less hydrocarbons (0,65%), whereas the EO extracted from the fruit in Thua Thien - Hue Province, Vietnam showed a lower amount of oxygen-containing components (78.8%) and a higher amount of hydrocarbons (21%) 44. Studies on hydrosols of Indian oregano (Origanum vulgare), caraway (Carum carvi), bergamot-mint (Mentha citrata) also led to similar results 19, 40, 41, 45, 46.
Hydrosols have been widely used in many regions of the world for food flavoring and medicinal purposes for a long time 2, 3. Moreover, the hydrophilic property makes hydrosols safe for skin. Since hydrosols are well-tolerated by the skin, they have thus been used in cosmetic products 47. Hydrosols of several spices and aromatic plants were demonstrated to possess high antifungal and antibacterial properties against a variety of bacteria and fungi48, 49, 50, 51. For example, hydrosols extracted from cardamom (Elettaria cardamomum), thyme (Thymus schimperi), and cinnamon (Cinnamon zeylanicum) showed the complete growth inhibition against Escherichia coli, Staphylococcus aureus and Salmonella typhi 49. The Satureja hortensis hydrosol 15% (v/v) was shown to have fungicidal effects, inhibiting 100% of mycelial growth of Rhizoctonia solani, Botrytis cinerea and Alternaria citri 48. However, hydrosol of Epilobium parviflorum exhibited weak antimicrobial activities towards tested Gram-negative bacteria (E. coli and Pseudomonas aeruginosa) and yeast (C. albicans), with MIC values of 70% (v/v); towards Gram-positive bacteria (S. aureus and Enterococcus faecalis), the MIC values were greater than 90% 47. In the present study, the L. cubeba fruit hydrosol exhibited strong antimicrobial activities towards the bacterium H. pylori (MIC of 10%, MBC of 30%) and the yeast C. albicans (MIC of 10%, MFC of 40%). The cells of H. pylori and C. albicans were killed completely after 24 and 18 hours of treatment with the 30% and 40% hydrosol, respectively.
There have been no data of antibacterial and antifungal activities of the hydrosol extracted from the L. cubeba fruits, yet the information of those of the L. cubeba fruit EO have been well-known. The L. cubeba fruit EO showed strong inhibitory effects on Gram-positive bacteria (Bacillus subtilis, E. faecalis, S. aureus) and Gram-negative bacteria (E. coli and P. aeruginosa), and Monilia albicans 33, and high growth-inhibiting activity towards methicillin-resistant S. aureus 31. In addition, the EO exhibited good fungicidal activities against Sclerotinia sclerotiorum, Thanatephorus cucumeris 35, Aspergillus flavus 52, and C. albicans 42. Bacteriostatic and bactericidal activities of the L. cubeba EO significantly depend on concentration and exposure time 32. The EO at concentration of 0.0625% (v/v) was able to prolong the growth lag phase of E. coli cells to approximate 12 hours, whereas the 0.125% (v/v) concentration killed the cells completely within 2 hours32.
The antibacterial and antifungal effects of plant EOs and their hydrosols have been attributed to their compositions. The richness in oxygenated monoterpenes, oxygenated sesquiterpenes, and phenolic compounds contributed to the strong antimicrobial activities of hydrosols 7, 53, 54. Phenolic compounds can interfere with microbial membranes, cell walls, or the action of microbial enzymes 2. Terpenoid compounds may disrupt the lipid structure and thus cause loss of membrane integrity, membrane protein functions (proton pumps and enzymes), and synthesis of cellular metabolites, leading to cell death 31, 55. The antimicrobial effects of the L. cubeba fruit hydrosol may be mainly due to the presence of citral (geranial and neral) 32, 56. Neral and geranial were found to be the two main components of lemongrass EO, which was demonstrated to have strong antibacterial activity against Gram-negative and Gram-positive bacteria 57 and resulted in a considerable decrease in the density of H. pylori in mouse stomach 24. Citral and sabinene were reported to exhibit strong anti-H. pylori activities 58. Citral was also shown to exhibit excellent activities against dermatophytes, thereby suggesting its potency as a fungicidal agent59. Furthermore, citral showed strong anti-fungal abilities towards pathogenic microbes E. coli, P. aeruginosa, S. aureus, and C. albicans, and was considered as a potential agent to control skin and mucosal infections60. The synergistic effects among major and minor components of the L. cubeba fruit hydrosol against H. pylori and C. albicans need to be further studied.
Conclusion
The present study revealed that the L. cubeba fruit hydrosol exhibited pronounced inhibitory and microbicidal effects against H. pylori and C. albicans. The results suggest that the L. cubeba fruit hydrosol warrants further study and could be developed as a potent antimicrobial product for treating C. albicans and H. pylori infections.
Abbreviations
ATCC: American Type Culture Collection
dEO: dissolved essential oil
EO: Essential oil
GC-MS: Gas chromatography-mass spectrometry
MALDI-TOF MS: Matrix-assisted laser desorption ionization-time of flight mass spectrometry
MIC: Minimal inhibitory concentration
MBC: Minimal bactericidal concentration
MFC: Minimal fungicidal concentration
Acknowledgments
Authors gratefully acknowledge the financial support from Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-YS.06-2015.17.
Author’s contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-YS.06-2015.17.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Fleisher
A.,
Fleisher
Z.,
Water-soluble fractions of the essential oils. Perfumer Flavorist.
1991;
16
(3)
:
37-41
.
View Article Google Scholar -
D'Amato
S.,
Serio
A.,
López
C.C.,
Paparella
A.,
Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control.
2018;
86
:
126-137
.
View Article Google Scholar -
Rao
B.R.,
Hydrosols and water-soluble essential oils of aromatic plants: Future economic products. Indian Perfum.
2012;
56
:
29-33
.
-
Cid-Pérez
T.S.,
Ávila-Sosa
R.,
Ochoa-Velasco
C.E.,
Rivera-Chavira
B.E.,
Nevárez-Moorillón
G.V.,
Antioxidant and antimicrobial activity of Mexican Oregano (Poliomintha longiflora) essential oil, hydrosol and extracts from waste solid residues. Plants.
2019;
8
(1)
:
22
.
View Article PubMed Google Scholar -
Inouye
S.,
Takahashi
M.,
Abe
S. ,
Inhibitory activity of hydrosols, herbal teas and related essential oils against filament formation and the growth of Candida albicans. Nippon Ishinkin Gakkai Zasshi.
2009;
50
(4)
:
243-251
.
View Article PubMed Google Scholar -
Özcan
M.,
Effect of spice hydrosols on the growth of Aspergillus parasiticus NRRL 2999 strain. J Med Food.
2005;
8
(2)
:
275-278
.
View Article PubMed Google Scholar -
Tabet-Zatla
A.,
Dib
M.E.A.,
Djabou
N.,
Ilias
F.,
Costa
J.,
Muselli
A.,
Antifungal activities of essential oils and hydrosol extracts of Daucus carota subsp. sativus for the control of fungal pathogens, in particular gray rot of strawberry during storage. J Essent Oil Res.
2017;
29
(5)
:
391-399
.
View Article Google Scholar -
Marshall
B.,
Warren
J.R.,
Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet.
1984;
323
(8390)
:
1311-1315
.
View Article Google Scholar -
Smith
S.M.,
O'Morain
C.,
McNamara
D.,
Helicobacter pylori resistance to current therapies. Curr Opin Gastroen.
2019;
35
(1)
:
6-13
.
View Article PubMed Google Scholar -
Den-Hoed
C.M.,
Kuipers
E.J.,
Helicobacter pylori infection. Hunter's Tropical Medicine and Emerging Infectious Diseases: Elsevier.
2020;
:
476-480
.
-
Atherton
J.C.,
Blaser
M.J.,
Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest.
2009;
119
(9)
:
2475-2487
.
View Article PubMed Google Scholar -
Negrei
C.,
Boda
D.,
The Mechanisms of action and resistance to fluoroquinolone in Helicobacter pylori Infection. In: Roesler. Trends in Helicobacter pylori Infection BM editor. IntechOpen.
2014;
:
349
.
View Article Google Scholar -
Calderone
R.A.,
Fonzi
W.A.,
Virulence factors of Candida albicans. Trends Microbiol.
2001;
9
(7)
:
327-335
.
View Article Google Scholar -
Mayer
F.L.,
Wilson
D.,
Hube
B.,
Candida albicans pathogenicity mechanisms. Virulence.
2013;
4
(2)
:
119-128
.
View Article PubMed Google Scholar -
Calderone
R.A.,
Clancy
C.J.,
Candida and candidiasis. American Society for Microbiology Press.
2011
.
View Article Google Scholar -
Williams
D.W.,
Jordan
R.P.,
Wei
X.Q.,
Alves
C.T.,
Wise
M.P.,
Wilson
M.J.,
Lewis
M.A.,
Interactions of Candida albicans with host epithelial surfaces. J Oral Microbiol.
2013;
5
(1)
:
22434
.
View Article PubMed Google Scholar -
Dąbrowska
M.,
Sienkiewicz
M.,
Kwiatkowski
P.,
Dąbrowski
M.,
Diagnosis and treatment of mucosa Candida spp. infections-a review article. In: Annales Universitatis Mariae Curie-Sklodowska, sectio C-Biologia.
2019;
73
(1)
:
61-68
.
View Article Google Scholar -
Rimbara
E.,
Fischbach
L.A.,
Graham
D.Y.,
Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol.
2011;
8
(2)
:
79
.
View Article PubMed Google Scholar -
Binh
T.T.,
Shiota
S.,
Nguyen
L.T.,
The incidence of primary antibiotic resistance of Helicobacter pylori in Vietnam. J Clin Gastroenterol.
2013;
47
(3)
:
233
.
View Article PubMed Google Scholar -
Mohammadi-Ghalehbin
B.,
Heravi
H. Javanpour,
Arzanlou
M.,
Sarvi
M.,
Prevalence and antibiotic resistance pattern of Candida spp. isolated from pregnant women referred to health centers in Ardabil, Iran. JAUMS.
2017;
16
(4)
:
409-421
.
-
Quek
C.,
Antimicrobial susceptibility and clarithromycin resistance patterns of Helicobacter pylori clinical isolates in Vietnam. F1000Research.
2016;
5
.
View Article PubMed Google Scholar -
Ali
S.M.,
Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrob.
2005;
4
(1)
:
20
.
View Article PubMed Google Scholar -
Bonifácio
B.V.,
Ramos
M.A.S.,
Silva
P.B.,
Bauab
T.M.,
Antimicrobial activity of natural products against Helicobacter pylori: a review. Ann Clin Microbiol Antimicrob.
2014;
13
(1)
:
54
.
View Article PubMed Google Scholar -
Ohno
T.,
Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter.
2003;
8
(3)
:
207-215
.
View Article PubMed Google Scholar -
Zida
A.,
Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J Mycol Med.
2017;
27
(1)
:
1-19
.
View Article PubMed Google Scholar -
Ngan
L.T.M.,
Moon
J.K.,
Shibamoto
T.,
Y.J. Ahn,
Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and-resistant strains of Helicobacter pylori. J Agr Food Chem.
2012;
60
(36)
:
9062-9073
.
View Article PubMed Google Scholar -
Chi
V.,
Dictionary of Vietnamese Medicinal Plants (New Edition). Hanoi, Vietnam: Medicine Publishing House.
2012
.
-
Ho
P.H.,
Cay Co Viet Nam. An Illustrated Flora of VietNam. Youth Publishing House, Ho Chi Minh City.
2000;
3
.
-
Loi
D.,
Glossary of vietnamese medicinal plants and drugs. Publishing House for Science and Technics, Hanoi.
2000
.
-
Hammid
S.A.,
Ahmad
F.,
Chemotype of Litsea cubeba Essential oil and its bioactivity. Nat Prod Commun.
2015;
10
(7)
.
View Article Google Scholar -
Hu
W.,
Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind Crops Prod.
2019;
130
:
34-41
.
View Article Google Scholar -
Li
W.R.,
Antibacterial activity and kinetics of Litsea cubeba oil on Escherichia coli. PLoS One.
2014;
9
(11)
.
View Article PubMed Google Scholar -
Wang
H.,
Liu
Y.,
Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem Biodivers.
2010;
7
(1)
:
229-235
.
View Article PubMed Google Scholar -
Liu
L.,
Inhibition of Litsea cubeba oil on biofilm initial formation stage of Candida albicans. Int J Lab Med.
2017;
38
(20)
:
2850-2851
.
-
Yang
Y.,
The fungicidal terpenoids and essential oil from Litsea cubeba in Tibet. Molecules.
2010;
15
(10)
:
7075-7082
.
View Article PubMed Google Scholar -
Lee
H.K.,
Growth inhibitory, bactericidal, and morphostructural effects of dehydrocostus lactone from Magnolia sieboldii Leaves on antibiotic-susceptible and-resistant strains of Helicobacter pylori. PloS one.
2014;
9
(4)
.
View Article PubMed Google Scholar -
Collin
G.,
Gagnon
H.,
Chemical composition and stability of the hydrosol obtained during the production of essential oils. III. The case of Myrica gale L., Comptonia peregrina (L.) Coulter and Ledum groenlandicum Retzius. Am J Essen Oil Nat Prod.
2016;
4
(1)
:
7-19
.
-
Garneau
F.X.,
Collin
G.,
Gagnon
H.,
Chemical composition and stability of the hydrosols obtained during essential oil production. I. The case of Melissa officinalis L. and Asarum canadense L. Am J Essent Oil Nat Prod.
2014;
2
:
54-62
.
-
Garneau
F.X.,
Collin
G.,
Gagnon
H.,
Chemical composition and stability of the hydrosols obtained during essential oil production. II. The case of Picea glauca (Moench) Voss., Solidago puberula Nutt., and Mentha piperita L. Am J Essent Oil Nat Prod.
2014;
2
:
29-35
.
-
Rivera
L.L.,
Water soluble fractions of caraway (Carum carvi L.) essential oil. B Latinoam Caribe Pl.
2010;
9
(6)
:
495-500
.
-
Verma
R.,
Analysis of the hydrosol aroma of Indian oregano. Med Aromat Plants.
2012;
1
(112)
.
View Article Google Scholar -
Saikia
A.K.,
Screening of fruit and leaf essential oils of Litsea cubeba Pers. from north-east India-chemical composition and antimicrobial activity. J Essent Oil Res.
2013;
25
(4)
:
330-338
.
View Article Google Scholar -
Si
L.,
Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules.
2012;
17
(6)
:
7057-7066
.
View Article PubMed Google Scholar -
Son
L.C.,
Analysis of the essential oils from five Vietnamese Litsea species (Lauraceae). J Essent Oil Bear Plants.
2014;
17
(5)
:
960-971
.
View Article Google Scholar -
N.Merad-Boussalah
Chemical Composition and Biological Activities of Essential Oil and Hydrosol Extract from Aerial Parts of Cynoglossum cheirifolium L. from Algeria. J Essent Oil Bear Plants.
2020;
23
(1)
:
97-104
.
View Article Google Scholar -
Verma
S.K.,
Chemical composition and antimicrobial activity of bergamot-mint (Mentha citrata Ehrh.) essential oils isolated from the herbage and aqueous distillate using different methods. Ind Crops Prod.
2016;
91
:
152-160
.
View Article Google Scholar -
Smigielski
K.B.,
Prusinowska
R.,
Krosowiak
K.,
M
M. Sikora,
Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula angustifolia). J Essent Oil Res.
2013;
25
(4)
:
291-299
.
View Article Google Scholar -
Boyraz
N.,
Özcan
M.,
Antifungal effect of some spice hydrosols. Fitoterapia.
2005;
76
(7-8)
:
661-665
.
View Article PubMed Google Scholar -
Hussien
J.,
Assessment of the antimicrobial effects of some Ethiopian aromatic spice and herb hydrosols. Int J Pharmacol.
2011;
7
(5)
:
635-640
.
View Article Google Scholar -
Ozturk
I.,
Decontamination of iceberg lettuce by some plant hydrosols.. LWT.
2016;
74
:
48-54
.
View Article Google Scholar -
Shen
X.,
Chemical composition, antibacterial and antioxidant activities of hydrosols from different parts of Areca catechu L. and Cocos nucifera L. Ind Crop Prod.
2017;
96
:
110-119
.
View Article Google Scholar -
Li
Y.,
Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind Crop Prod.
2016;
80
:
186-193
.
View Article Google Scholar -
Belabbes
R.,
Chemical variability, antioxidant and antifungal activities of essential oils and hydrosol extract of Calendula arvensis L. from western Algeria. Chem Biodivers.
2017;
14
(5)
:
e1600482
.
View Article PubMed Google Scholar -
Voda
K.,
Boh
B.,
Vrtačnik
M.,
Pohleven
F.,
Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int Biodeterior Biodegradation.
2003;
51
(1)
:
51-59
.
View Article Google Scholar -
Sikkema
J.,
Bont
J.A.,
Poolman
B.,
Mechanisms of membrane toxicity of hydrocarbons. Microbiol Mol Biol Rev.
1995;
59
(2)
:
201-222
.
View Article Google Scholar -
Liu
T.T.,
Yang
T.S.,
Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. Int J Food Microbiol.
2012;
156
(1)
:
68-75
.
View Article PubMed Google Scholar -
Onawunmi
G.O.,
Yisak
W.A.,
Ogunlana
E.,
Antibacterial constituents in the essential oil of Cymbopogon citratus (DC.) Stapf. J Ethnopharmacol.
1984;
12
(3)
:
279-86
.
View Article Google Scholar -
Bergonzelli
G.,
Donnicola
D.,
Porta
N.,
Corthesy-Theulaz
I.,
Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob Agents Chemother.
2003;
47
(10)
:
3240-3246
.
View Article PubMed Google Scholar -
Wannissorn
B.,
Jarikasem
S.,
Soontorntanasart
T.,
Antifungal activity of lemon grass oil and lemon grass oil cream. Phytother Res.
1996;
10
(7)
:
551-554
.
View Article Google Scholar -
Usach
I.,
Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials.
2020;
10
(2)
:
286
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 6 (2020)
Page No.: 3819-3828
Published on: 2020-06-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7726 times
- Download PDF downloaded - 2056 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress