Collagenolytic activitiy in tissue extract of Parborlasia corrugatus from antarctic region
Abstract
Marine organisms have been recognized as rich sources of bioactive compounds with valuable biotechnology potential. Enzymes extracted from marine hydrobionts have gained much attention because of their unique quite specific properties that determined their profound applications in chemical, medical, food industries and molecular biology experiments. In this regard, our work focused on investigation of proteolytic potential of marine hydrobionts. At first, tissue extract of Antarctic hydrobiont Parborlasia corrugatus was separated by gel filtration chromatography on a Superdex-75 PG. Further zymography with using gelatin as substrate revealed the presence of clear band that can indicate about active enzymes. It had been shown the presence of collagenolytic activity in all eight fractions obtained after chromatographic separation of tissue extract. Trypsin-like (L-BApNA hydrolyzing) was found only in first fraction. Our results let us assume that P. corrugatus can be regarded as potential source of enzymes for practical use.
Introduction
Increasing the demand for and use of the products the active component of which is represented by hydrolytic enzymes determines the relevance of finding new alternative sources of hydrolytic enzymes. Unfortunately, the industrial application for food, cosmetic and pharmaceutical of mammalian proteases has recently been limited by the outbreak of some animals’ diseases or/and religious tradition. For this reasons many investigators have focused their attention on marine organisms importance of which as a source of new substances is growing. It should be noted that their survival in a very exigent, competitive and aggressive surrounding causes not only considerable structural and functional diversity of marine biologically active compounds but also the presence of enzymes that may be used in both basic research and industrial applications Fuchise et al., 2011Songklanakarin, 2008. So, as evolutionary precursors of proteases of warm-blooded animals, hydrolases of cold-water marine invertebrates are characterized a number of unique quite specific properties. Thus they have significantly higher total proteolytic activity towards native protein substrates, broader substrate specificity and lower activation energy for catalysis compared with proteases from mammalian or microbial sources. In addition enzymes from cold-water hydrobionts are more active at low temperatures than those from mammals, thermophylic organisms and plants Peck et al., 2004. All this makes the prospect of their use in several industrial applications, such as in certain food processing operations that require low processing temperatures. In terms of practical application enzymes posses collagenolytic activity are very perspective - these protease can be applied in chemical, medical, food industries, biotechnology and as well as for molecular biology experiments.
Taking into account given above, the aim of present study was to investigate the proteolytic activity in tissue extract of P. corrugatusfrom Antarctic region for its potential biomedical and industrial applications.
Material and methods
In our study we used a frozen mass of marine hydrobionts of Antarctic region - Antarctic nemertean P. corrugatus. The samples were collected in terms of the expedition and were brought to the laboratory in iced condition. Frozen mass of hydrobiont was weighed and homogenized with sequential addition of liquid nitrogen and extraction buffer – 0.1 M Na-phosphate buffer containing 0.15 M NaCl, 0.15 mM EDTA, 2 мМ PMSF and 0.1% triton X-100, pH 7.4. After homogenization (60 sec) the homogenate was centrifuged 60 min, 10000 g at 4ºC. Supernatant was selected and lyophilized. Thus obtained primary material can be stored for a long time at a temperature of 20°C without losing the functional properties of proteins and peptides.
The protein concentration was determined according to the method of Bradford, 1976 with bovine serum albumin as standard. Zymography was done according to the method Ostapchenko et al., 2011. Separating gel was polymerized in the presence of gelatin (1 mg/ml). Zymography was performed on native- PAGE. Represented zymogram is typical for the series of repeated experiments. Collagenolytic activity was measured with help of native collagen type I. The extent of collagen digestion was determined using the Moore and Stein colorimetric ninhydrin process Moore, 1954. Trypsin-like activity was measured using N-α-benzoyl- L-Arg-p-nitroanilide (L-BApNA) as substrate Xavier et al., 2005.The amount of p-nitroaniline liberated from BApNA was assessedby the increase in absorbance at 405 nm. Three replicates were always used to quantify enzyme activity.
The statistical analysis of the obtained results was performed using the methods of variation statistics. Whereby differences P < 0.05 were deemed reliable.
Results and discussion
Some features of living conditions of marine organisms that are strongly affected by number of factors, in particular, the salinity of ocean waters, low light or its absence, hydrostatic pressure, temperature fluctuations provide unique physicochemical, toxicological and biological characteristics of metabolites from marine organisms and their difference from natural compounds from warm-blooded animals.
Therefore, improvement and development of new methodological approaches for isolation of marine hydrobionts metabolites, study their structure and spectrum of biological activity, without any exaggeration, can be considered as one of the priority direction of scientific and technological progress development.
Given the above the primary objective of our work was to optimize the conditions for the fractionation of tissues extracts of aquatic organisms, as well as to identify and characterize the individual protein fractions associated with the manifestation of a certain type of enzyme activity.
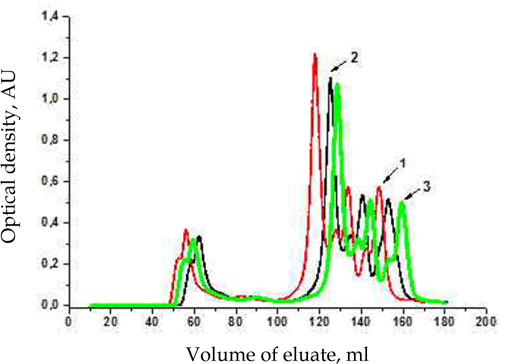
Since the molecular weights of proteins and peptides representing a specific biotechnological interest, usually, are in the range from 5 to 120 kDa for the fractionation of tested extract, we applied the method of gel filtration chromatography with using as a chromatographic matrix Superdex-75 PG. Taking into account the dependence of the efficiency of protein and peptide fractions separation on a number of factors such asa chromatographic matrix Superdex-75 PG. Taking into account the dependence of the efficiency of protein and peptide fractions separation on a number of factors such as volume flow rate, gel filtration buffer, appropriate protein concentration in the sample, etc. it is important to correctly choose the conditions for proper chromatographic separation. Selection of optimal conditions for fractionation was conducted on example of Antarctic scallop Adamussium colbecki. According to our results, the optimal separation of protein-peptide composition of analyzed extract was observed under the following conditions - flow rate is 1 ml/min ( Figure 1 ), gel filtration buffer is 50 mM tris-HCl, pH 7.4 with 0.15 M NaCl ( Figure 2 ). Using of this buffer or buffers with equivalent ionic strength allows to reduce nonspecific interactions between the proteins being separated and the chromatographic matrix, that is important for efficient and standardized separation of protein samples.
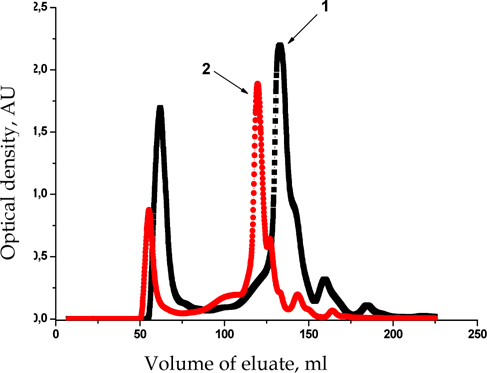
It should be noted that the dissolution of the samples in buffers with different ionic strength had no effect on the character of protein fractions separation ( Figure 3 ).
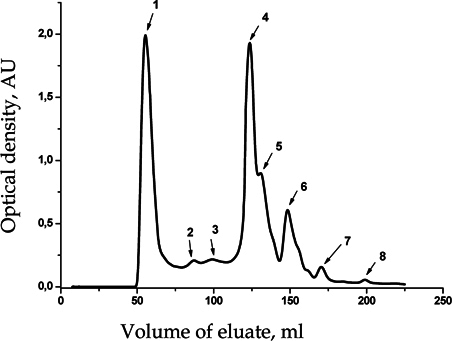
Separation of tissue extract of P. corrugatus conducted in compliance with all of the above parameters yielded eight peaks ( Figure 4 ), that correspond to protein and peptide fractions in the molecular weight range 3 to 70 kDa and performs best between 8 and 50 kDa.
The presence in studied samples the proteins with different molecular weight may indicate the existence of functionally active molecules with different enzymatic activities. Therefore, it is appropriate to test the obtained fractions for the presence of active enzymes. Zymographic technique was used to detect proteolytic enzymes following electrophoretic separation in gels. This method is based on a SDS-polyacrylamide gel, which co-polymerized with the protein substrate that is degraded by the proteases restored during the incubation period in the enzyme reaction buffer after the electrophoretic separation.
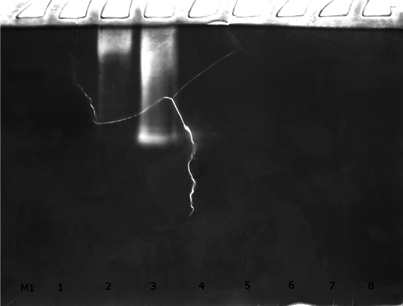
The results of identification of proteolytic activity in eight protein fraction obtained after chromatographic separation of P. corrugatus tissue extract is shown at Figure 5 . It should be emphasized that preparation of separating gel solution in the presence of gelatin allows to identify enzymes belong to family of proteases.
The appearance at gelatin zymogram expressed clear bands in 2, 3 and 4 peaks confirms the presence in analyzed fractions functionally active proteolytic enzymes. Using of gelatin as a substrate allows us to assume that these proteolytic activities can be associated with gelatinases or/and collagenases Wilkesman and Kurz, 2009, that are structurally and functionally very similar to the vertebrate enzymes and can belong to the group of serine proteases as well as to the group of metalloproteases.
According to the literature in tissues of marine invertebrates was found a number of hydrolytic enzymes which take part in several fundamental processes, such as extracellular matrix remodeling, embryonic development, cell growth and differentiation and also in defense mechanisms thus highlightening their intriguing and unexpected functional importance in invertebrate life Flood et al., 2000Ghamari et al., 2014Salamonea, 2012Songklanakarin, 2008.
Taking into account the feeding habits of representatives of Nemertea we can suppose that studied hydrobiont may containdigestive enzymes capable to hydrolyze native collagen.
It should be noted that collagenolytic enzymes of marine hydrobionts have the ability to cleave the triplehelix of collagen under conditions which do not denature the protein. In this respect, they are similar to collagenases of higher animals which play a role in the physiological remodeling of connective tissues. There is, however, a definite difference in the catalytic mechanism between the above digestive collagenolytic erzymes and vertebrate collagenases - the latter enzymes catalyze the cleavage of the substrate predominantly in one particular point and substantially incapable to hydrolyze large soluble fragments Mott and Werb, 2004whereas collagenolytic proteases of hydrobionts can cleave native collagen in many points Rudenskaya et al., 2004.
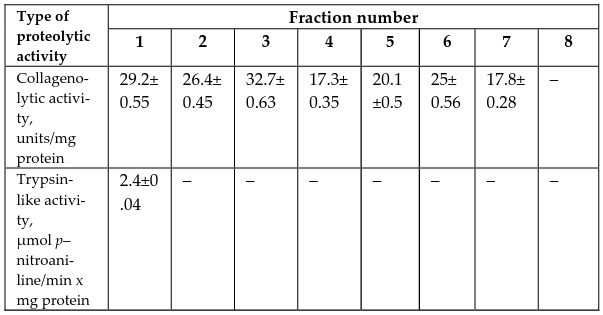
On this basis, the next stage of our work was devoted to analyze the protein fractions obtained after gel filtration chromatography of tissue extract of P. corrugatus on the presence of collagenolytic activity. According to obtained results summarized in ( Table 1 ) this type of proteolytic activity was found to be present in seven examined peaks. We revealed maximum collagenolytic activity in the peak, which correspond to third fraction that, in general, is entirely consistent with the data of zymography about the presence of the most expressed clear band at third trek of zymogram.
Based on literature data Park et al., 2002Ramundo and Gray, 2009 and our own results we can conclude that collagenolytic activity in 1-3 peaks may indicate the presence in tissue extracts of P. corrugatus collagenases, the molecular weights of which are in the average range of 30 to 70 kDa, whereas activity in 4-7 peaks may be associated with low molecular weight collagenolytic serine proteases. So, a number of author note that in contrast to high-molecular bacterial and tissue collagenases of higher animals which molecular weights are found to vary from 30 to 150 kDa collagenolytic enzymes of marine invertebrates, are complex of isoenzymes with molecular weights up to 36 kDa Kim et al., 2002Salamonea, 2012.
Thus, our findings may indirectly indicate the presence in tissues extract of investigated hydrobiont enzymes belonging to the serine collagenolytic proteases as well as to metallocollagenases, that, of course, require more detailed studies using appropriate inhibitors.
As trypsins are principal digestive proteinases in most hydrobionts Fuchise et al., 2011, in addition to collagenolytic activity we studied trypsin-like activity. The hydrolytic activity was assessed using the synthetic substrates L-BApNA. Taking into account that LBApNA is also suitable substrate for cysteine proteinase we used E-64 - aninhibitor of this family of enzymes that has enabled us to estimate serine protease activity, particularly, activity of trypsin-like enzymes. Results of measurement of proteolytic activity in tissue extract of P. corrugatusare summarized in Table 1 . According to obtained data trypsin-like activity towards amidolytic substrate was poorly expressed. So, proteolytic enzymes having trypsin-like activity were found only in first peak and no detectable activity was observed in 2-8 peaks. Such low value of enzymatic activity providing support for the presence in tissue extracts of P. corrugatus trypsin-like enzymes with other substrate specificity, e.g. esterase.
Summarizing obtained results it should be noted that collagenolytic activity in investigated extract is more expressed in compared to trypsin-like activity. Established differences can be caused by both physiological characteristics of this hydrobiont and its taxonomic affiliation.
It is important to emphasize that our study is only at preliminary stage - further identification of enzymes with use of different substrates and specific inhibitors should provide more detail information about structure functional and catalytic characteristics of investigated proteases.
Conclusion
In summary, obtained results indicate the presence of collagenolytic and trypsin-like activities in tissue extract of Antarctic hydrobiont P. corrugatus. Further researches in direction of purification and characterization of individual proteases from marine hydrobionts should provide more detail information about its function, catalytic mechanism and will contribute to successful industrial application of investigated enzymes according to its properties.
References
-
M.
Bradford.
A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry.
1976;
72
:
248-254
.
-
J.
Flood,
J.
Mayne,
J.J.
Robinson.
Identification and characterization of gelatin-cleavage activities in the apically located extracellular matrix of the sea urchin embryo. Biochemistry and Cell Biology.
2000;
78
:
455-462
.
-
T.
Fuchise,
H.
Sekizaki,
H.
Kishimura,
S.
Klomklao,
S.
Nalinanon,
S.
Benjakul,
B.-S.
Chun.
Simple Preparation of Pacific Cod Trypsin for Enzymatic Peptide Synthesis. Journal of Amino Acids.
2011;
2011
:
1-8
.
-
M.
Ghamari,
V.
Hosseininaveh,
A.
Darvishzadeh,
K.
Talebi.
Biochemical characterisation of the tissue degrading enzyme, collagenase, in the spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae). Journal of Plant Protection Research.
2014;
54
.
-
S.-K.,
P.-J.
Park,
J.-B.
Kim,
F.
Shahidi.
Journal of Biochemistry and molecular biology. 2002;
35
:
165-171
.
-
S.
Moore,
W.H
Stein.
A modified ninhydrin reagent for the determination of amino acids and related compounds. Journal of Biological Chemistry.
1954;
211
:
907-913
.
-
J.D.
Mott,
Z.
Werb.
Regulation of matrix biology by matrix metalloproteinases. Current Opinion in Cell Biology.
2004;
16
:
558-564
.
-
L.
Ostapchenko,
O.
Savchuk,
N.
Burlova-Vasilieva.
Enzyme electrophoresis method in analysis of active components of haemostasis system. Advances in Bioscience and Biotechnology.
2011;
02
:
20-26
.
-
P.-J.
Park,
S.-H.
Lee,
H.-G.
Byun,
S.-H.
Kim,
S.-K.
Kim.
Journal of Biochemistry and molecular biology. 2002;
35
:
576-582
.
-
L.S.
Peck,
K.E.
Webb,
D.M.
Bailey.
Extreme sensitivity of biological function to temperature in Antarctic marine species. Functional Ecology.
2004;
18
:
625-630
.
-
J.
Ramundo,
M.
Gray.
Collagenase for Enzymatic Debridement. Journal of.
2009;
Wound
:
Ostomy and Continence Nursing 36, S4-S11
.
-
G.N.
Rudenskaya,
Y.A.
Kislitsin,
D.V.
Rebrikov.
BMC Structural Biology. 2004;
4
:
2
.
-
M.
Salamonea,
A.
Cuttittab,
G.
Seiditac,
S.
Mazzolad,
F.
Bertuzzie,
C.
Ricordif,
G
Ghersig.
Characterization of collagenolytic/proteolytic marine enzymes. Chemical Engineering Transactions.
2012;
27
:
1-6
.
-
S.K.
Songklanakarin.
Digestive proteinases from marine organisms and their applications. J Sci Technology 30.
2008;
(1)
:
37-46
.
-
J.
Wilkesman,
L.
Kurz.
Protease Analysis by Zymography: A Review on Techniques and Patents. BIOT.
2009;
3
:
175-184
.
-
L.P.
Xavier,
M.G.
Almeida Oliveira,
R.N.C.
Guedes,
A.V.
Santos,
S.G.
De Simone.
Trypsin-like activity of membrane-bound midgut proteases from Anticarsia gemmatalis (Lepidoptera: Noctuidae). Eur J Entomol.
2005;
102
:
147-153
.
Comments

Downloads
Article Details
Volume & Issue : Vol 2 No 9 (2015)
Page No.: 354-358
Published on: 2015-09-10
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4730 times
- Download PDF downloaded - 1613 times
- View Article downloaded - 3 times
 Biomedpress
Biomedpress