Abstract
Introduction: Ginger has had a long history of use in healthcare in Asian countries, including Vietnam. Internationally, there have been a few studies done to assess the anticancer activity and potency of ginger. In this study, we investigated the activity (cytotoxicity) of various ginger root extracts that were produced by different preparation methods on human hepatocellular carcinoma cell line (HepG2 cell line).
Methods: Ginger (Zingiber officinale Roscoe) root extract were extracted from the fresh ginger roots by three methods, which included maceration (G1), Soxhlet (G3), and ultrasonic (G5) methods. The extracts were then evaluated for cytotoxic activity on HepG2 hepatocellular carcinoma cell line based on IC50 calculation. The most potent extract was then evaluated for its cytotoxicity by Propidium iodide (PI) staining method and microscopic examination by fluorescence microscopy for both 2D and 3D culture conditions. After treatment, cells were also stained with Annexin-V to assess the apoptosis-inducing ability of the extract.
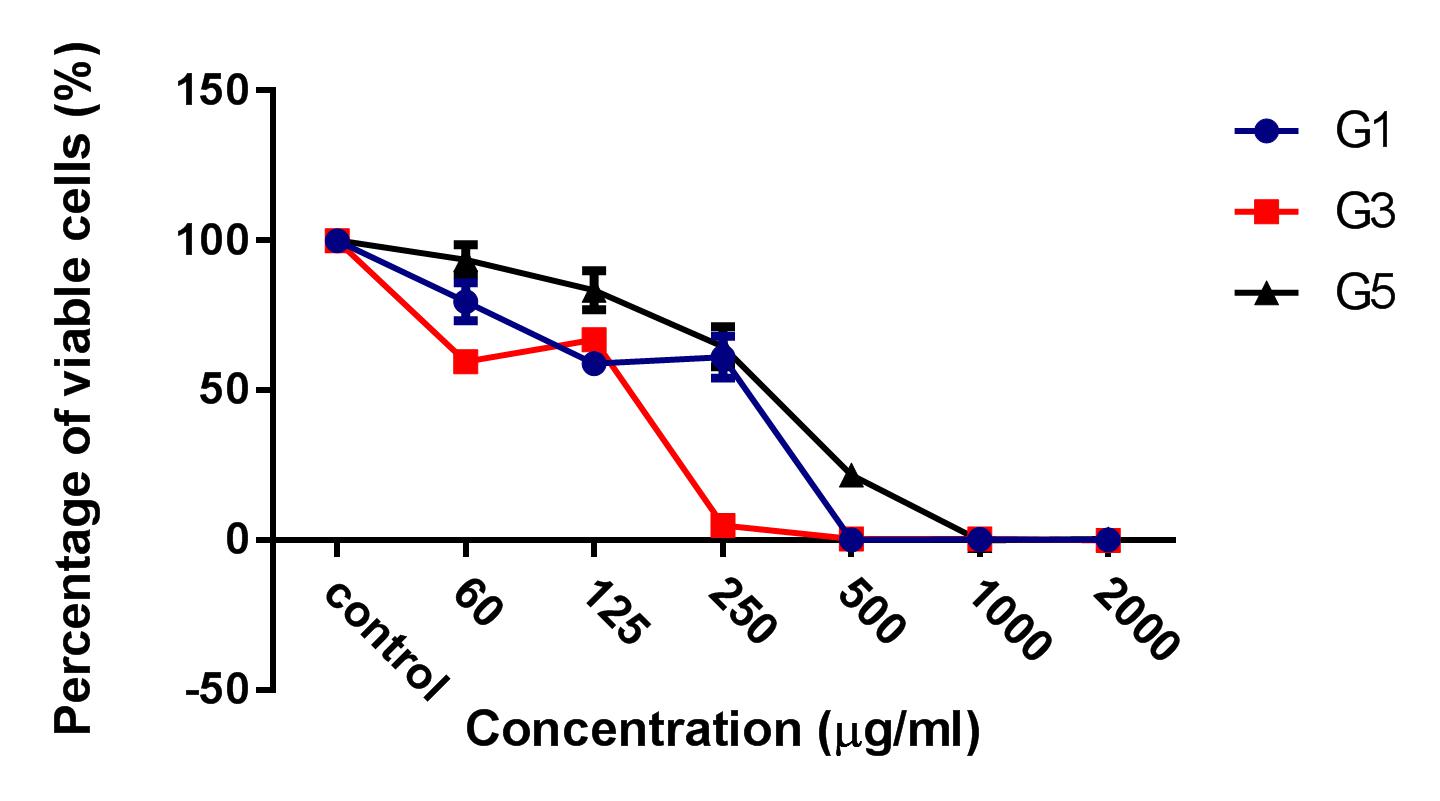
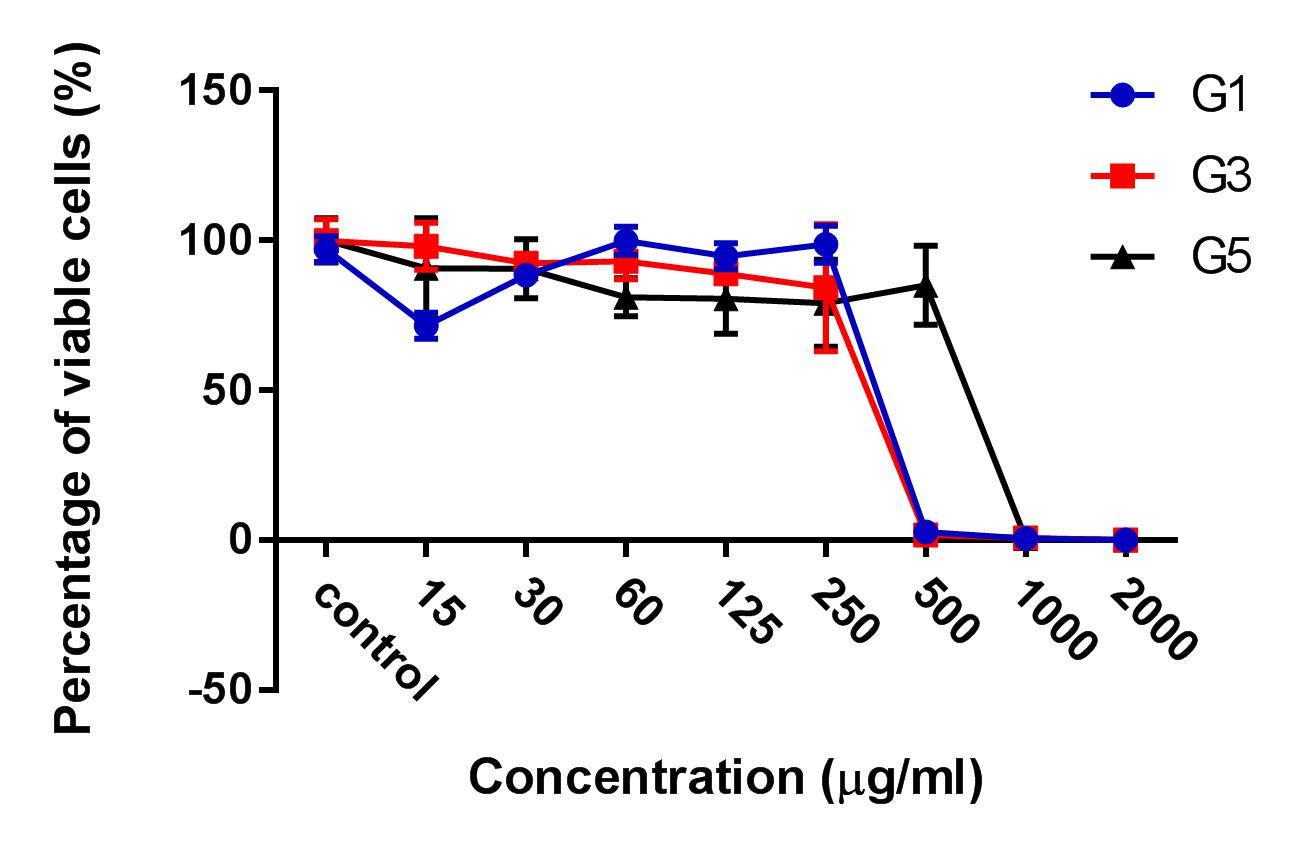
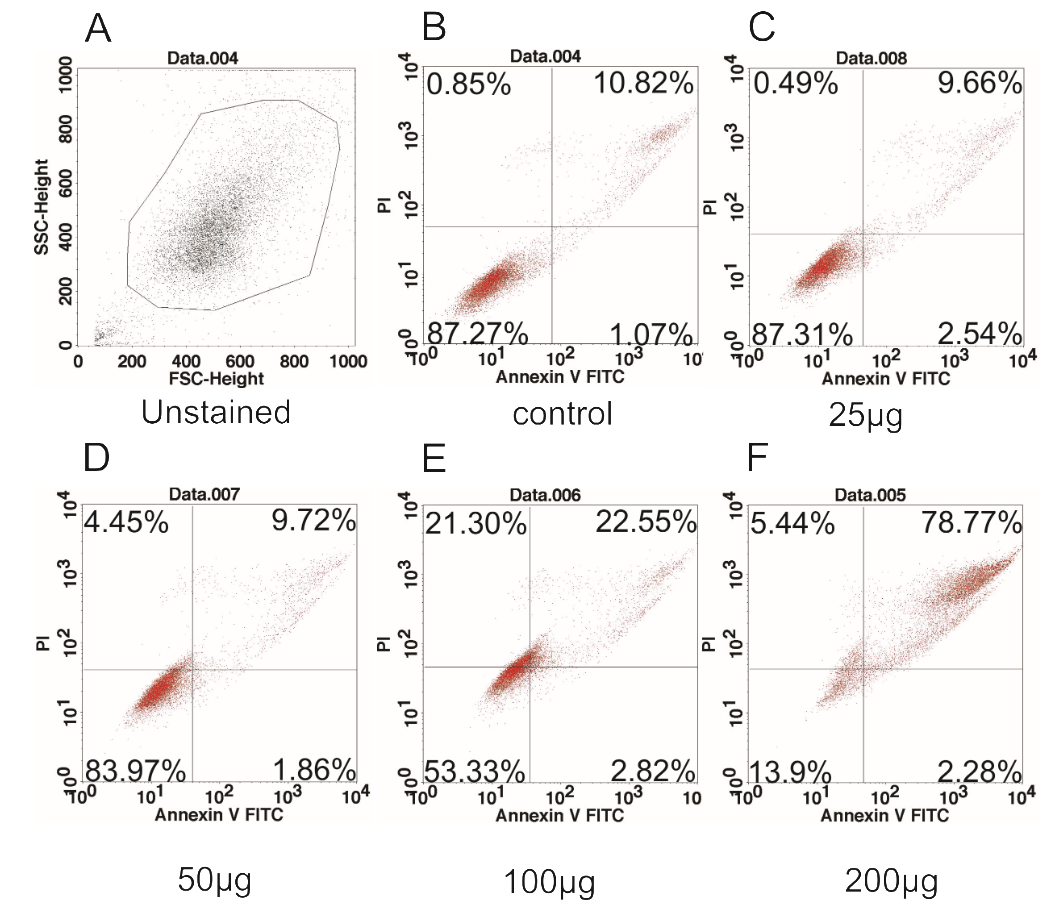
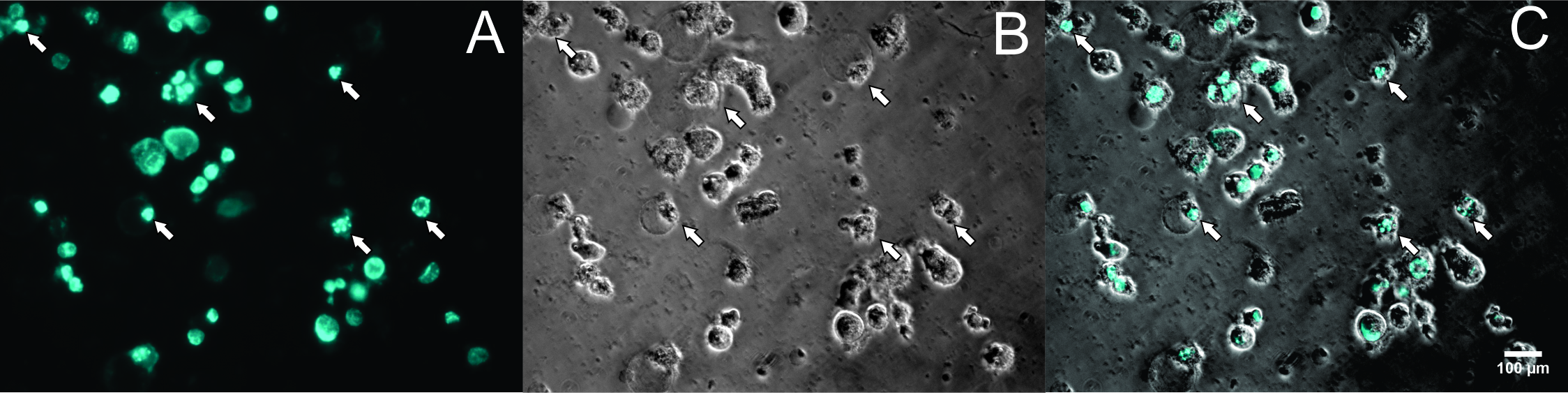
Results: The results showed that the ginger root extract prepared by the Soxhlet method (G3) had the strongest activity, and showed significant differences compared to extracts from methods G1 and G5, on the hepatocellular carcinoma cell lines cultured in both 2D and 3D conditions. Indeed, the IC50 value of ginger root extract of the G3 was 83.3 +/- 0.9189 ug/ml, compared to 159 +/- 7.6 ug/ml of G1, and 284 +/- 5.116 ug/ml of G5 in 2D culture latform; the IC50 value was 228 +/- 33.52 ug/ml for G3 compared to 341+/- 3.93 ug/ml for G1, and 603.7 +/- 56.33 ug/ml for G5 in 3D culture platform. The results of PI staining of cells in 2D and 3D culture conditions showed that ginger root extract killed HepG2 cells in a concentration-dependent manner. The nuclei of HepG2 cells in 2D culture showed nuclear fragmentation when treated with ginger root extract; this is one of the apoptosis indicators. Moreover, Annexin-V staining results showed that HepG2 cells underwent apoptosis when treated with ginger root extract.
Conclusion: The results of the study show that ginger root extract prepared by the Soxhlet method induced strong cytotoxicity of hepatocellular carcinoma cells cultured in both 2D and 3D conditions, through induction of apoptosis. The evidence suggests that ginger is a potential agent for anticancer therapy. It is necessary to perform further research to isolate compounds from fresh ginger root extract by the Soxhlet method to evaluate the mechanism of anti-tumor activity.
Introduction
Traditional medicine has played a necessary role in improving patient health for thousands of years. Traditional herbal treatments are beneficial in that they are a complementary remedy to reduce complications during treatment and can reduce tumor growth1,2. In particular, herbal medicines and their derivatives have been documented to cure diseases via their antioxidant, anti-inflammatory, anti-diabetic, and anticancer properties3.
Ginger root (Zingiber officinale) is a member of the family Zingiberaceae. Since ancient times, ginger has been widely used as a spice and condiment in food and beverages in many Eastern countries4. In oriental medicine, ginger has also been regarded primarily as a remedy for digestive disorders, such as colic, gastritis, vomiting, dyspepsia, nausea, and diarrhea5. Furthermore, ginger root extract and its pungent components (such as gingerol, paradol, and shogaol3,6) have potential anti-inflammatory7, antioxidant8, and anticancer9,10,11 activities.
Inflammatory disorders are caused not only by infectious agents, such as parasites, bacteria, and viruses, but also by physical and chemical factors, including heat, acid, smoke and physical damage12,13. Recently, many non-steroidal drugs are commonly used for anti-inflammatory treatment, but side effects and gastric ulcers are some drawbacks3. Gingerol, shogaol, and structurally-related substances in ginger were evidenced to inhibit inflammation by down-regulating the synthesis of pro-inflammatory cytokines, including NFκB-regulated genes14, TNF-α, IL-1, and IL-815.
Antioxidant properties play a significant role in balancing the production and neutralization of free radicals and oxidative stress16. Ginger extract and its constituents show antioxidant effects and protect macromolecules from damages induced by free radicals and oxidative stress3. Gingerol and gingerol-related compounds16,17 paradol18, zingerone19,20, and ginger flavonoids21 are the main ingredients of ginger extract and have been proven to have high antioxidant effects.
Tumor development and formation progress through various stages, including genetic and metabolic changes22. Ann M. Bode et al. provided evidence that [6]-gingerol can inhibit VEGF, and [6]-paradol can induce apoptosis in transformed cells23. Other investigations have demonstrated that [6]-gingerol and constituents of ginger play a significant role in suppression of transformation and hyper-proliferation that relate to stages of carcinogenesis, metastasis, and tumorigenesis24,25. Furthermore, Nidhi Nigam et al. pointed out that [6]-gingerol increased benzo[a]pyrene (B[a]P)-suppressed p53 and Bax levels while decreasing Bcl-2 expression25.
This research study was performed to optimize the method for ginger root extraction and investigate its anticancer activity. Alamar Blue staining was used to analyze cell viability and IC50 value. Furthermore, flow cytometry was performed with Annexin-V/ Propidium Iodide (PI) staining, and DNA fragmentation was observed with Hoechst 33342 staining to detect apoptosis induction.
Materials-Methods
Ginger roots
Ginger root (Zingiber officinale) within the age of 7-8 months was collected from Daklak, a province in the highlands of Vietnam. The whole root of fresh ginger was used in this study.
Extracts from ginger roots by Soxhlet, Sonication, and Maceration
Extracting the ginger root by Soxhlet
Twenty gram of the fresh ginger root was grinded. Then, 100ml of absolute ethanol solvent (Merck, Germany) was added into the flask together with the ginger root. The process was conducted at 78.4oC for 12 hours, with 5-6 heat cycles in a heating mantle (MTOP, Republic of Korea) for 1 hour.
Extracting the ginger root by Sonication
100 g of the fresh ginger root was grinded and placed in a flask. Then, 500 ml of absolute ethanol was placed into the Ultrasonic Cleaner machine (JPS, CA, USA). The ultrasound process was conducted at a temperature of 15oC for 30 minutes. The mixture after the ultrasound was filtered. The process was continued by refining 2-3 times with Whatman filter paper (Sigma Aldrich, MO, USA) to remove residue completely.
Extracting the ginger root by Maceration
100 g of the fresh ginger root was soaked in 500 ml of absolute ethanol in Becher for 72 hours. After 72 hours, the whole mixture was filtered to remove residue.
Cell culture (2D)
Human hepatocarcinoma cell line HepG2 was purchased from ATCC (Manassas, VA, USA). The cell line was grown in DMEM/F12 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma Aldrich, St. Louis, MO, USA) and 5% antibiotic (Thermo Fisher Scientific). HepG2 cells were put into a 5% CO2 humidified atmosphere at 37˚C. All the experiments were performed in triplicate.
Cell culture (3D)
The HepG2 cell line was seeded in a Hanging drop 96-well plate (3D Biomatrix, Ann Arbor, MI, USA) with DMEM/F12 medium supplemented with 10% FBS and 5% antibiotic. The cell density was 5000 cells per well (in 50 μl volume). The cells tended to cluster together and form spheroids after three days.
Apoptosis assay
Cells were labeled with 3 μl of Annexin V-FITC (Miltenyi Biotec, Germany) and 3 μl of Propidium Iodide (PI) (Miltenyi Biotec) in 500 μl of binding buffer for 15 min to detect apoptotic and necrotic cell death using a FACSCalibur Flow Cytometer (BD, Franklin Lake, NJ, USA). Data were analyzed by Cellquest software (BD).
Cytotoxicity assay on 2D and 3D cultures
After 2 days for 2D culture and 3 days for 3D culture, the cells in 96-well plates were treated with the ginger root extract by Soxhlet, Sonication, and Maceration. The concentration of these extracts was diluted 2-fold serially from 2000 µg/ml to 15 µg/ml. Following 48h of incubation, the cells were evaluated for viability by staining with Alamar Blue and reading the measurements through a DTX 880 machine (Beckman Coulter, CA, USA).
Nuclei staining assay
To detect cell death after treatment with the extracts, cells were stained with 10 μl of PI. After that, the cells were incubated in a 5% CO2 humidified atmosphere at 37˚C. Cell images were captured after 1 h by a Zeiss Axio Imager fluorescence microscope (Zeiss, Germany).
Statistical analysis
The raw data from the cytotoxicity assay on the 2D and 3D culture cells were calculated for IC50 values. Accordingly, based on the untreated group (negative control) with 100% live cells, the calculation would detect the concentration of 50% of cells. The experiments were repeated three times and processed statistically with 95% confidence intervals. Statistical analysis was done using the GraphPad Prism software (GraphPad Software, La Jolla, CA, USA).
Results
Ginger root extracts inhibited HepG2 pro liferation in 2D and 3D models
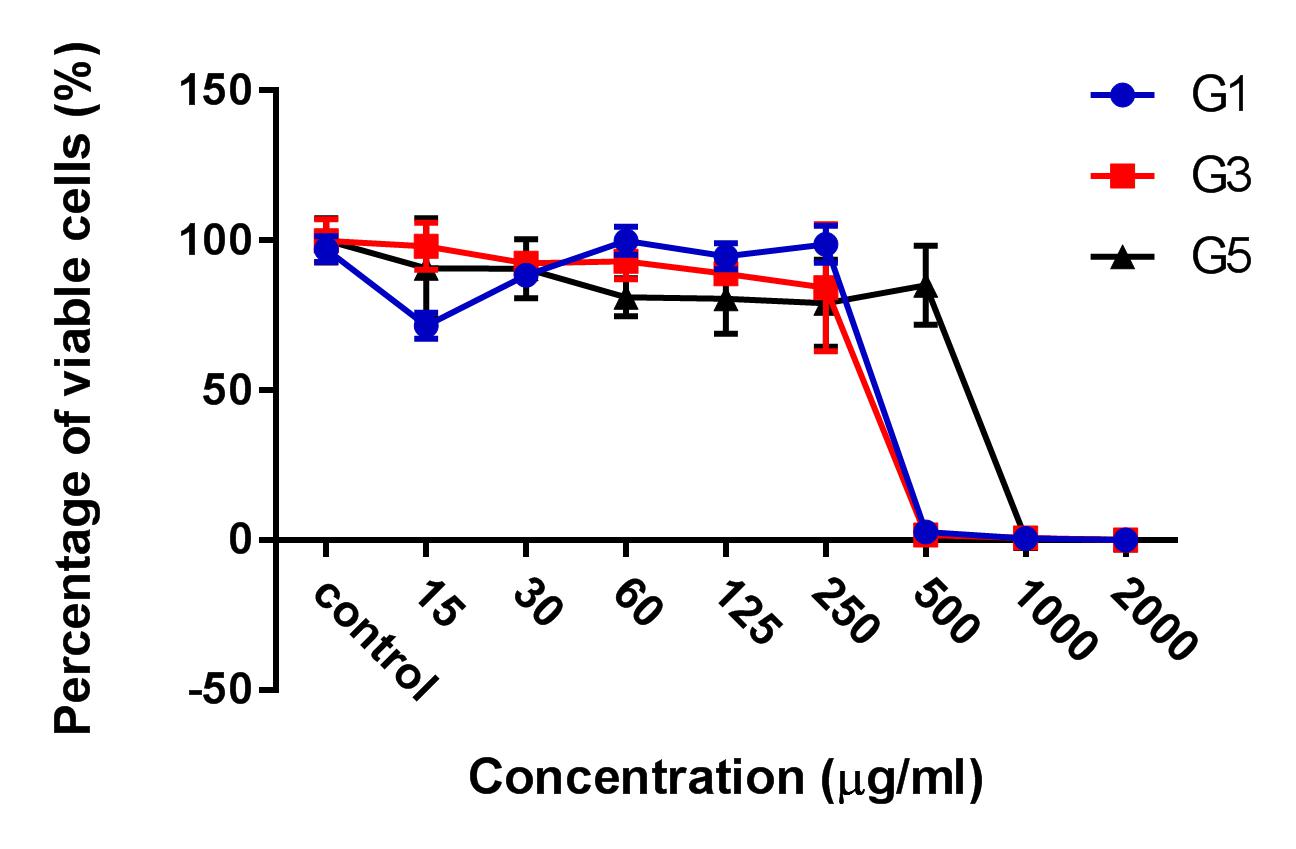
HepG2 cells were treated with 3 kinds of ginger root extracts (from Maceration (G1), Soxhlet (G3), and Sonication (G5) methods) in 2D cell culture conditions to select for the extract with the highest cytotoxicity on HepG2 cells. The results showed that Soxhlet extract had the strongest cytotoxic activity on HepG2 with an IC50 of 83.3 ± 0.9189 µg/ml, while the IC50 values of the extract from methods of Maceration and Sonication were 159 ± 7.6 µg/ml and 284 ± 5.116 µg/ml, respectively. These results showed that the ginger root extract from Soxhlet showed the stronger antitumor cytotoxicity against HepG2.
After the treatment of HepG2 with ginger root extract in 2D conditions (Figure 1), we continuously used ginger root extract to treat HepG2 in 3D conditions (Figure 2). The results showed no significant differences in IC50 between the ginger root extracts from the different preparation methods; the IC50 of G3 extract was 228 ± 33.52 µg/ml, while IC50 of G1 was 341 ± 3.93 µg/ml and of G5 was 603.7 ± 56.33 µg/ml. The data show that G3 has more potent cytotoxicity compared with G1 and G5.
Ginger root extracts induced apoptosis of HepG2 cells in 2D culture
As aforementioned, from the results of IC50 of ginger root extract on 2D and 3D culture conditions, it turned out that G3 (the ginger root extract by Soxhlet method) had the strongest anti-toxicity on HepG2 in both 2D and 3D culture conditions. Therefore, we selected G3 to evaluate its ability to induce apoptosis of HepG2 cells. We treated HepG2 cells with G3 extract at the concentrations of 200 µg/ml, 100 µg/ml, 50 µg/ml, 25 µg/ml, or 0 µg/ml (control). The results demonstrated G3 extract induced apoptosis in a dose-dependent manner. At the concentration of 200 µg/ml, almost all cells underwent apoptosis, with 78.77% in late apoptosis.
To perform Annexin-V staining assay, cells were stained with PI and Hoechst 33342 and observed under microscopy. The results showed that at the concentration of 200 µg/ml (Figure 3D), 400 µg/ml (Figure 3E), and 800 µg/ml (Figure 3F), almost all cells were stained with PI (red color) (Figure 4). At the high microscope magnification, the nuclei of cells treated with G3 extract at 200 µg/ml were condensed and disintegrated (Figure 5). These kinds of features indicate that the cells underwent apoptosis.
Death cells of HepG2 spheroids after treatment with ginger root extract
We also evaluated how the G3 extract impacted HepG2 spheroids in 3D culture conditions. After culturing HepG2 cells in 3D and following spheroid development, the spheroids were treated with G3 extract and compared with G1 and G5 extracts (Figure 6). The HepG2 spheroids showed to break apart and to stain with PI at the concentration 250 µg/ml of Ginger extract, while untreated spheroids still remained the initial shape. At the concentrations of 500 and 1000 µg/ml, the G1, G2, and G3 extracts all had an impact on the HepG2 spheroids. These results demonstrate that G3 has strong activity on HepG2 spheroids.
Ginger extract triggers apoptosis of HepG2 spheroids
We then selected the extract G3 to evaluate its ability to induce apoptosis by flow cytometry. We observed that even in untreated spheroids, there was about 30% of cells which underwent apoptosis (Figure 7). This was likely due to the core of HepG2 spheroids, where there is a lack of oxygen and nutrients. However, after spheroids were treated with G3 extract, the apoptotic populations increased to 40% at the IC50/2; 77.04% at IC50; almost all cells were in late apoptosis at IC50 x 2 and IC50 x 4. These results demonstrated the impact of G3 extract on HepG2 spheroids. Together with the ability to break the HepG2 spheroids apart, the G3 extract induced the HepG2 cells in spheroids to undergo apoptosis.
Discussion
Phytochemical extracts from fruits and vegetables are increasingly being shown to exert potent antioxidant and anti-proliferative effects. It is widely becoming appreciated that chemo-preventative agents offer superior potential in the long term over chemotherapeutic agents. Life style and dietary habits have been identified as major risk factors, particularly in cancer growth and progression.
Ginger root is extensively used in the form of a fresh paste or dried powder to flavor food and beverages. Recently, a study showed that treatment with 2 mg/ml of ginger with 31 mg/ml of gelam honey inhibited the growth of most HT29 cells and induced apoptosis in a dose-dependent manner, with the combined treatment yielding the highest apoptosis rate. This was caused by the downregulation of gene expression of Akt, mTOR, and Raptor, while cytochrome C and caspase 3 genes where shown to be upregulated26 Recently, ginger root extract also showed anti-leukemia and anti-drug resistant effects27.
In the present study, we show that ginger extracted by Soxhlet has stronger cytotoxic activity on HepG2 cells than extracts from Maceration and Sonication. The ginger extract could induce apoptosis of HepG2 cell lines cultured in both 2D and 3D conditions. Sonication and Maceration methods were not strong enough to break down the cell wall of the ginger plant to release certain active agents, but the Soxhlet method was capable of that. Recently, some studies have shown that ginger polysaccharides induced cell cycle arrest and apoptosis of HepG2 through up-regulation of Bax, caspase-3, and p53, and down-regulation of Bcl-228. We observed the typical apoptotic morphological changes in HepG2 cells, including cell shrinkage and detachment, and nuclear condensation and fragmentation in 2D culture conditions of HepG2. This was in accordance with a study by Elkady et al.29 which demonstrated that ginger extract induced the upregulation of p53 and p21, which inducing down-regulation of cyclin D1 and cyclin-dependent kinanse-4 expression, resulting in G2/M2 arrest.
We firstly demonstrated the anti-tumor effect of ginger extract in 3D conditions. The extract induced HepG2 spheroids to break part from the original round shape. We also found that ginger extract induced cells in the HepG2 spheroids to undergo apoptosis. This might have caused the cells to lose their cell-to-cell contact, resulting in the disassembly of the HepG2 spheroids.
6-shogaol is a major active constituent of dietary ginger that has been demonstrated to inhibit cell proliferation in osteosarcoma30, lung cancer31, and colon cancer32, through increasing apoptosis pathways which lead to a decrease of expression levels of anti-apoptotic proteins (e.g. survivin and Bcl-2) and increasing pro-apoptotic proteins (e.g. Bax). In HepG2 cell lines, 6-shogaol and 6-gingerol (two active components in ginger) showed anti-invasion and anti-metastasis properties in vitro33.
Taken together, ginger extract possesses constituents that are able to block proliferation and induce the death of HepG2 cells in both 2D and 3D culture conditions.
Conclusion
Ginger has a long history of use in the diet and medicine of humans. This study contributes further evidence of the potency of ginger root extract. We show that ginger possesses potent cytotoxicity on HepG2 cells grown in 2D or 3D, suggesting its potential benefit as an anticancer agent in disease treatment. Some studies on the constituents of ginger have further documented its ability to induce apoptosis in vitro. Further studies of ginger root extract, and its active constituents, in vivo using animal models, are needed to ascertain its anticancer potency prior to its application for human disease.
Abbreviations
G1: ginger root extract by Maceration method
G3: ginger root extract by Soxhlet method
G5: ginger root extract by Ultrasonic method
NFκB: nuclear factor kB
TNF- α: tumor necrosis factor alpha
IL: interleukin
VEGF: vascular endothelial growth factor
Conflict of intetest
We have no conflict of interest to declare.
Author Contributions
Sinh Nguyen, Phuc Van Pham conceived and planned the experiments. Phuc Hong Vo, Thao Duy Nguyen, Nghia Minh Do, Bao Huu Le, Duong Thuy Dinh performed the experiments. Kiet Dinh Truong, Sinh Nguyen analysed the data. Sinh Nguyen wrote the manuscript. Phuc Pham and Kiet Dinh Truong revised the manuscript.
Acknowlegdment
This work was supported by the Vietnam National University, Ho Chi Minh City, Vietnam, under grant A2015-18-01.
References
-
Lichota
A.,
Gwozdzinski
K.,
Anticancer Activity of Natural Compounds from Plant and Marine Environment. International Journal of Molecular Sciences.
2018;
19
(11)
:
3533
.
View Article PubMed Google Scholar -
Yin
S.-Y.,
Therapeutic applications of herbal medicines for cancer patients. . Evidence-Based Complementary and Alternative Medicine.
2013;
2013
:
302426
.
View Article Google Scholar -
Rahmani
A.H.,
Shabrmi
F.M.,
Aly
S.M.,
Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. International Journal of Physiology, Pathophysiology and Pharmacology.
2014;
6
(2)
:
125-36
.
PubMed Google Scholar -
Park
E.J.,
Pezzuto
J.M.,
Botanicals in cancer chemoprevention. Cancer and Metastasis Reviews.
2002;
21
(3-4)
:
231-55
.
View Article PubMed Google Scholar -
Srivastava
K.C.,
Mustafa
T.,
Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders. Medical Hypotheses.
1992;
39
(4)
:
342-8
.
View Article PubMed Google Scholar -
Govindarajan
V.S.,
Connell
D.,
Ginger\textemdashchemistry, technology, and quality evaluation: part 1. Critical Reviews in Food Science and Nutrition.
1982;
17
(1)
:
1-96
.
PubMed Google Scholar -
Hudson
E.A.,
Fox
L.H.,
Luckett
J.C.,
Manson
M.M.,
Ex vivo cancer chemoprevention research possibilities. Environmental Toxicology and Pharmacology.
2006;
21
(2)
:
204-14
.
View Article PubMed Google Scholar -
Jeyakumar
S.,
Nalini
N.,
Menon
V.P.,
Antioxidant activity of ginger (Zingiber officinale Rosc) in rats fed a high fat diet. Medical Science Research.
1999;
27
(5)
:
341-4
.
-
Benzie
I.F.,
Wachtel-Galor
S.,
CRC press 2011.
View Article Google Scholar -
Borrelli
F.,
Capasso
R.,
Aviello
G.,
Pittler
M.H.,
Izzo
A.A.,
Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstetrics and Gynecology.
2005;
105
(4)
:
849-56
.
View Article PubMed Google Scholar -
Shukla
Y.,
Singh
M.,
Cancer preventive properties of ginger: a brief review. Food and Chemical Toxicology.
2007;
45
(5)
:
683-90
.
View Article PubMed Google Scholar -
Ohshima
H.,
Tatemichi
M.,
Sawa
T.,
Chemical basis of inflammation-induced carcinogenesis. Archives of Biochemistry and Biophysics.
2003;
417
(1)
:
3-11
.
View Article PubMed Google Scholar -
Mashhadi
N.S.,
Ghiasvand
R.,
Askari
G.,
Hariri
M.,
Darvishi
L.,
Mofid
M.R.,
Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. International Journal of Preventive Medicine.
2013;
4
:
36-42
.
PubMed Google Scholar -
Habib
S.H.,
Makpol
S.,
Abdul Hamid
N.A.,
Das
S.,
Ngah
W.Z.,
Yusof
Y.A.,
Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (S{&}{#}x00E3;o Paulo).
2008;
63
(6)
:
807-13
.
View Article PubMed Google Scholar -
Jung
H.W.,
Yoon
C.H.,
Park
K.M.,
Han
H.S.,
Park
Y.K.,
Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food and Chemical Toxicology.
2009;
47
(6)
:
1190-7
.
View Article PubMed Google Scholar -
Shyur
L.F.,
Antioxidant properties of extracts from medicinal plants popularly used in Taiwan. Int J Appl Sci Eng.
2005;
3
(3)
:
195-202
.
-
Masuda
Y.,
Kikuzaki
H.,
Hisamoto
M.,
Nakatani
N.,
Antioxidant properties of gingerol related compounds from ginger. BioFactors (Oxford, England).
2004;
21
(1-4)
:
293-6
.
View Article PubMed Google Scholar -
Chung
W.Y.,
Jung
Y.J.,
Surh
Y.J.,
Lee
S.S.,
Park
K.K.,
Antioxidative and antitumor promoting effects of [6]-paradol and its homologs. Mutation Research.
2001;
496
(1-2)
:
199-206
.
View Article PubMed Google Scholar -
Shin
S.G.,
Kim
J.Y.,
Chung
H.Y.,
Jeong
J.C.,
Zingerone as an antioxidant against peroxynitrite. Journal of Agricultural and Food Chemistry.
2005;
53
(19)
:
7617-22
.
View Article PubMed Google Scholar -
Aeschbach
R.,
Löliger
J.,
Scott
B.C.,
Murcia
A.,
Butler
J.,
Halliwell
B.,
Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food and Chemical Toxicology.
1994;
32
(1)
:
31-6
.
View Article PubMed Google Scholar -
Rahman
S.,
Salehin
F.,
Iqbal
A.,
In vitro antioxidant and anticancer activity of young Zingiber officinale against human breast carcinoma cell lines. BMC Complementary and Alternative Medicine.
2011;
11
(1)
:
76
.
View Article PubMed Google Scholar -
Arshad
R.,
Expressional evaluation of androgen receptor in transitional cell carcinoma of urinary bladder patients. British Journal of Medicine and Medical Research.
2011;
1
(4)
:
233-8
.
View Article Google Scholar -
Bode
A.M.,
Ma
W.Y.,
Surh
Y.J.,
Dong
Z.,
Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Research.
2001;
61
(3)
:
850-3
.
PubMed Google Scholar -
Lee
H.S.,
Seo
E.Y.,
Kang
N.E.,
Kim
W.K.,
[6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. The Journal of Nutritional Biochemistry.
2008;
19
(5)
:
313-9
.
View Article PubMed Google Scholar -
Nigam
N.,
George
J.,
Srivastava
S.,
Roy
P.,
Bhui
K.,
Singh
M.,
Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B[a]P-induced mouse skin tumorigenesis. Cancer Chemotherapy and Pharmacology.
2010;
65
(4)
:
687-96
.
View Article PubMed Google Scholar -
Wee
L.H.,
Morad
N.A.,
Aan
G.J.,
Makpol
S.,
Wan Ngah
W.Z.,
Mohd Yusof
Y.A.,
Mechanism of Chemoprevention against Colon Cancer Cells Using Combined Gelam Honey and Ginger Extract via mTOR and Wnt/β-catenin Pathways. Asian Pacific Journal of Cancer Prevention.
2015;
16
(15)
:
6549-56
.
View Article PubMed Google Scholar -
Rahimi Babasheikhali
S.,
Rahgozar
S.,
Mohammadi
M.,
Ginger extract has anti-leukemia and anti-drug resistant effects on malignant cells. Journal of Cancer Research and Clinical Oncology.
2019;
145
(8)
:
1987-98
.
View Article PubMed Google Scholar -
Wang
Y.,
Wang
S.,
Song
R.,
Cai
J.,
Xu
J.,
Tang
X.,
Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. International Journal of Biological Macromolecules.
2019;
123
:
81-90
.
View Article PubMed Google Scholar -
Elkady
A.I.,
Abu-Zinadah
O.A.,
Hussein
R.A.,
Crude Flavonoid Extract of Medicinal Herb Zingibar officinale Inhibits Proliferation and Induces Apoptosis in Hepatocellular Carcinoma Cells. Oncology Research.
2017;
25
(6)
:
897-912
.
View Article PubMed Google Scholar -
Fan
J.,
Yang
X.,
Bi
Z.,
6-Gingerol inhibits osteosarcoma cell proliferation through apoptosis and AMPK activation. Tumour Biology.
2015;
36
(2)
:
1135-41
.
View Article PubMed Google Scholar -
Hsu
Y.L.,
Hung
J.Y.,
Tsai
Y.M.,
Tsai
E.M.,
Huang
M.S.,
Hou
M.F.,
6-shogaol, an active constituent of dietary ginger, impairs cancer development and lung metastasis by inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in tumor-associated dendritic cells. Journal of Agricultural and Food Chemistry.
2015;
63
(6)
:
1730-8
.
View Article PubMed Google Scholar -
Hwang
J.S.,
Lee
H.C.,
Oh
S.C.,
Lee
D.H.,
Kwon
K.H.,
Shogaol overcomes TRAIL resistance in colon cancer cells via inhibiting of survivin. Tumour Biology.
2015;
36
(11)
:
8819-29
.
View Article PubMed Google Scholar -
Weng
C.J.,
Wu
C.F.,
Huang
H.W.,
Ho
C.T.,
Yen
G.C.,
Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Molecular Nutrition {&}amp; Food Research.
2010;
54
(11)
:
1618-27
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 11 (2019)
Page No.: 3433-3442
Published on: 2019-11-22
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 20495 times
- Download PDF downloaded - 2343 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress