Abstract
Introduction: Panax vietnamensis Ha et Grushv. (Ngoc Linh ginseng) – a new species recently discovered in Vietnam – has received much interest due to its rich content of saponins, including those unknown. This study assessed the effects of the Ngoc Linh ginseng extract fractions on proliferation and differentiation of cultured mouse neural stem cells.
Methods: Whole brains were harvested from E13.5-14 Swiss mouse fetuses. Isolated cells were floating seeded to form spheroid bodies. Neurospheres were treated with one in fractions of ethanol 200-500 mg/mL, or nbutanol 200 mg/mL, or aqueous 200-500 mg/mL for 5 days. Neural stem cells could persistently generate secondary spheres. Neurospheres strongly expressed nestin, CD24 and deriving cells could differentiate into the GFAP-positive astrocyte-like cells.
Results: Ginseng fractions significantly promoted neurosphere growth rate. Particularly, 200 mg/mL ginseng ethanol fraction significantly increased the neurosphere size (28.00 +/- 3.00%, p<0.0001) not showing degeneration to the 5th day. However, n-butanol and aqueous fraction could not sustain the sphere structure. Ginseng ethanol fraction also elevated in the G2/M proportion (28.73+/-0.45%, p<0.0001), up-regulated proliferation mRNA ki67 (4.605+/-6.48 fold-change, p<0.05), cycA1 (12.61+/-4.65 fold-change, p<0.0001), cycD1 (22.47+/-8.18 fold-change, p<0.0001), cycC (9.53+/-2.63 fold-change, p<0.0001) compared with those of the n-butanol or aqueous fraction-treated neurospheres. Shorten G0/G1 phase (47.08 +/-0.16, p<0.0001), up-regulation of sox2 (71.25+/-27.24 fold-change, p<0.0001) mRNA levels indicated self-renewal effect of the ginseng ethanol fraction; however, those of n-butanol and aqueous fraction-treated neurospheres suggested an inhibiting effect on the cell proliferation.
Conclusion: Panax vietnamensis extract fractions had a positive effect on the proliferation of cultured neural stem cells. The ethanol fraction at 200 mg/mL could significantly promote the growth rate while still sustained the integrity of treated spheres.
Introduction
In the Northeast and East Asian countries like Vietnam, Korea, and China, ginseng has been used thousands of years to enhance human health. Panax ginseng saponins were indicated improve Parkinsonian progress on animal models, cognitive performance of Alzheimer’s patients and traumatic brain injuries 1,2,3 due to regulating the neurotrophic factor-associated pathways4,5,6. Ginsenosides could promote the differentiation of neural stem cells7, enhancing the neuronal fate in cultured adipose-derived stem cells8. A significant source of ginseng saponins comes from popular species like P. ginseng C. A. Meyer, P. notoginseng, and P. quinquefolium. Recently, a new ginseng species – Panax vietnamensis – was found in Vietnam. New ginsenosides in P. vietnamensis were shown to ameliorate depression, neuronal oxidative stress and improve the cognitive performance in the mouse model9,10,11,12,13. However, these studies have poorly showed the effect of ginseng extracts on the in vitro neural stem cells.
Proliferating cells were discovered first in the rat brain by Altman, J. and G.D. Das14. Subsequently, neural stem cells (NSCs) from both animals and humans have been extensively studied and characterized both in vivo and in vitro15,16. In mammals, NSCs exist in both adult and embryonic brains at different developmental stages17. NSCs could differentiate into three functional cell types of the nervous system. Over the past decade, there has been a rising interest in the 3D culturing method for drug screening due to its mimicking the stem cell niche in the body18,19. Originally introduced by Reynolds and Weiss, the neurosphere culturing method has become a convenient model for screening pharmaceutical properties of substances on neural stem cells because it reduces the differentiation possibility compared to adherent NSCs20,21. In this study, we investigate the potential effects of P. vietnamensis extracts on cultured neurospheres. The proliferation and differentiation of neural stem cells were access to show the effects of P. vietnamensis extracts.
Materials-Methods
Animal and experimental design
This study was approved by our institutional ethical committee (Laboratory of Stem cell Research and Application, University of Science, VNU-HCM). Healthy, E13.5-15.5 pregnant Swiss mice were kept in a stable environment of 12 hours light-dark cycle in the Microventilation cage system (THREE-SHINE Inc., Korea) with ad libitum access to food and water and acclimated for 1 week before the operation.
Plant material and preparation
Five years of age Panax vietnamensis was provided by the Center of Ginseng and Medicinal materials, National Institute of Medicinal Materials (NIMM), Ho Chi Minh City, Vietnam. Crude extract of Panax vietnamensis was prepared following the same method previously published22. In brief, whole root and rhizome of the plant was air-dried and powdered. Firstly, ginseng powder was percolatively extracted using 96%, 48%, 24%, and 0% ethanol (Merck, USA), respectively. Next, the extract solutions would be evaporated at low-pressure and lyophilized to yield the crude ethanol extract (shortly regarded as “the ethanol fraction”). Lipid in the extract was eliminated by ethyl ether. Next, ethyl ether was discarded from the product and water-saturated n-butanol was added. The n-butanol was collected and lyophilized to give the n-butanol fraction. Deinonized water was added to the remaining solution, next gathered and lyophilized to yield the aqueous fraction.
Neural stem cells (NSCs) isolation and culture
The NSCs isolation and culture methods in this study were repeated those in our previous study23 with reference to the method described by Reynolds et al. and Zheng, X.-S., et al.17,24. E13.5-15 pregnant mice were deep anesthetized by 100 mg/kg of ketamine, and 16mg/kg of xylazine and cervical dislocated. Fetal brains were isolated and homogenized into sterile PSBA solution. Brain pieces were digested with 0.025% trypsin 0.02% EDTA solution for 10 minutes at 37oC. Trypsin inhibitor (Sigma- Aldrich, St Louis, MO) was used to stop the digestion. Single cells were collected through a 70 µm Falcon® cell strainer. About 2.106 cells was suspended in 5 mL of basal NSC medium (serum-free DMEM/F12 high glucose, containing 30 µg/mL EGF, 30 µg/mL bFGF, 500 IU/mL heparin, 5 mg/mL insulin, 1 mg/mL transferrin, 0.01mg/ml gentamicin) (all purchased from Sigma Aldrich, St Louis, MO), supplemented with 1X N-2 and 1X B-27 (Gibco™, ThermoFisher Scientific, USA). Cells were seeded upon the agarose-covered 25cm2 culture flask (Corning, USA) to prevent adhesion and cultured at 37oC, 5% of CO2. Medium was changed every 3 days.
Sub-culture and sphere formation assay
At confluence, all neurospheres or cell clumps were digested by 0.025% trypsin 0.02% EDTA solution for 10 minutes at 37oC. Cell pellet was collected and re-suspended in 5 mL of basal NSC medium.
For sphere formation assay, ~1000 single cells from neurospheres at passage 4th were seeded into 24-well plate. Formation of new spheres was recorded.
Immunocytochemistry
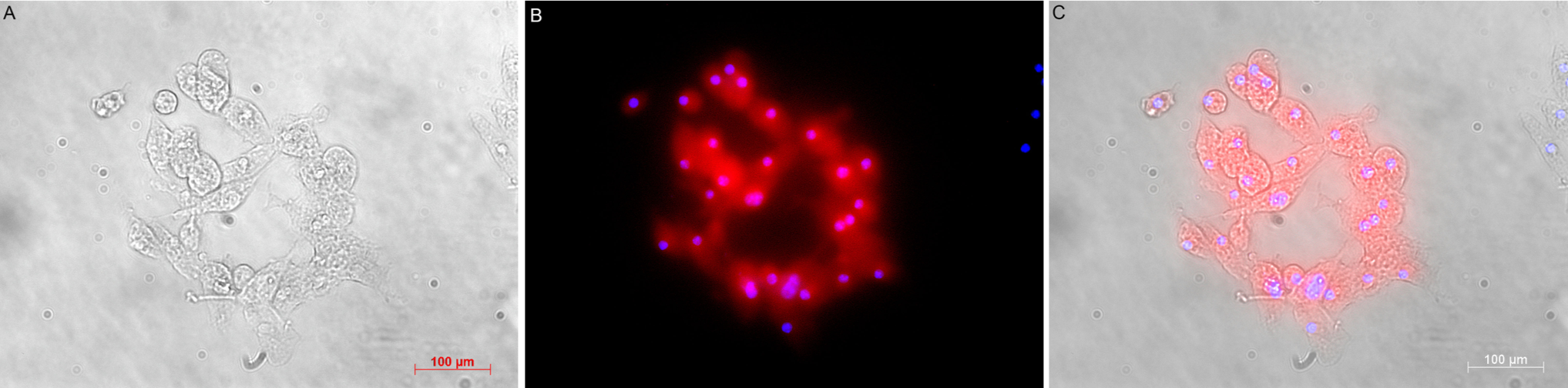
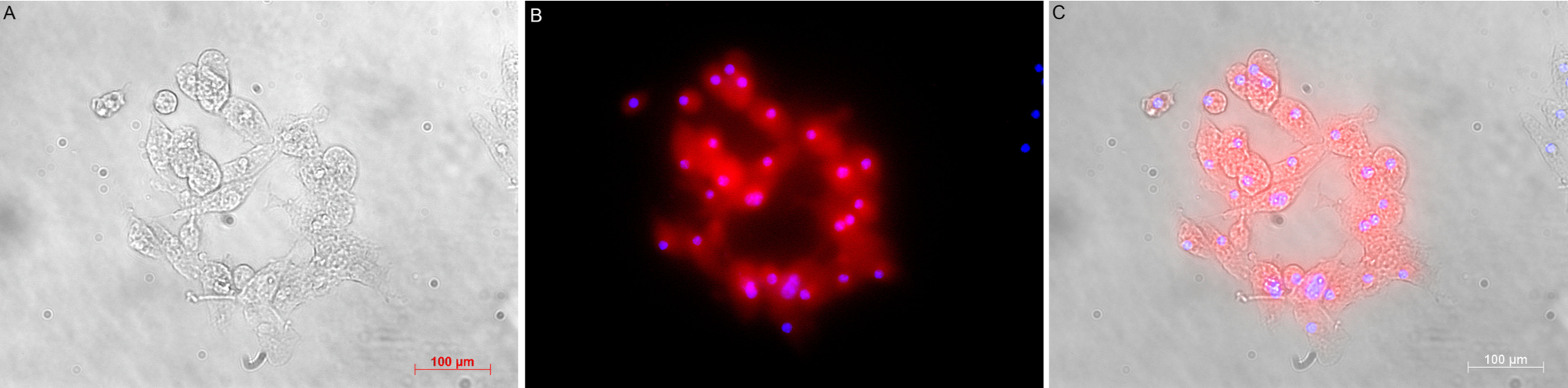
To examine Nestin expression, passage 4th neurospheres were collected, fixed in 1 mL of 1X FCM fixation buffer at RT, 30 mins and ice-cold, 5 mins 1X FCM permeabilization buffer (Santa Cruz Biotechnologies, USA). The sphere was incubated with 1st rabbit anti-mouse nestin antibody (1: 200 N5413, Sigma Aldrich, Singapore), then FITC-conjugated 2nd anti-Rb antibody (1:5000 ab6717, Abcam Singapore). Nuclei were stained with Hoescht 33342.
To examine GFAP expression, single cells from spheres were cultured in 2% FBS, EGF-free and bFGF-free basal NSC medium. Culture surface was covered with 50 μg/mL poly-L-Lysine to promote adhesion. After 10 days, spheres were fixed in FCM fixation buffer at RT for 5 minutes before being incubated with 1st rabbit anti-mouse GFAP antibody (1:100 ab16997, Abcam, Singapore) and rhodamine-conjugated 2nd anti-Rb antibody (4 μg/mL #31670 ThermoFisher Scientific, USA).
Ginseng treatments
For proliferation assay, 300µm-diameter neurospheres (n=10 spheres/each treatment) were used in ginseng treatment. The fraction was added to the basal medium with one of the concentrations 50, 100, 200 or 500 µg/mL). Basal NSC medium with or without 5 μg/mL nerve growth factor – NGF Sigma Aldrich, St Louis, MO) was used as the negative and positive control, respectively. Diameters of the neurospheres were recorded every day for 5 days. For differentiation assay, the neurospheres were first collected and transferred to an EGF- and bFGF-free basal NSC media which was supplemented with 200 µg/mL of ethanol, or n-butanol, or aqueous ginseng fraction. After 5 days, treated neurospheres were subjected to cell cycle analysis and gene expression.
Flow cytometry
Neurospheres were dissociated by 0.025% trypsin, 0.02% EDTA for 10 minutes at 37oC. One million cells were incubated with 0.25 μg FITC anti-mouse CD24 Antibody (Clone M1/69 BioLegend®). CD24 expression was analyzed by the FACSCalibur flow cytometer Biosciences and CellQuest Pro software (BD Biosciences, USA).
To analyze the cell cycle phase, cells were fixed with FCM fixation buffer (RT, 30 minutes) and ice-cold FCM permeabilization buffer (5 minutes), treated with 550 U/mL RNase A (Thermo Fisher Scientific, USA) at 37oC in 30 minutes. The cells were stained with 50 µg/mL PI (BD Biosciences, USA) at 37oC, no-light for 20-30 minutes. The DNA content was analyzed by the FACSCalibur flow cytometer BD Biosciences and CellQuest Pro software.
Quantitative RT-PCR
Total neurosphere RNA was extracted using Easy-BLUE Total RNA Extraction Kit (iNtRON Biotechnology, South Korea). Real-time RT-PCR analyses were performed using Brilliant III Ultra-Fast SYBR® Green qPCR Master Mix (Agilent, USA). Expression of cell cycle (ki67, cycA1, cycD1, cycC) and NSC markers (map2, gfap, mbp, sox2) genes were evaluated using Mastercycler® Ep Realplex (Eppendorf, Germany). Levels of expression were analyzed using Livak-method (2-ΔΔCt).
| Gene | Primer sequence (5’ – 3’) | Genes |
| gapdh | F: AAGTTGTCATGGATGACCR: TCACCATCTTCCAGGAGC | NM_001289726.1 |
| ki-67 | F: GCAGGAAGCAACAGATGAGAAGCCR: GCTCAGGTGATACATGCCTCCTGC | NM_001081117.2 |
| cycA1 | F: GTTTCCCCAATGCTGGTTGAR: AACCAAAATCCGTTGCTTCCT | NM_001305221.1 |
| cycD1 | F: CCAGAGGCGGATGAGAACAAR: ATGGAGGGTGGGTTGGAAAT | NM_007631.2 |
| cycC | F: CAGGACATGGGCCAGGAAR: TCCGTCCTGTAGGTATCATTCACTATC | NM_001290420.1 |
| map2 | F: GGCACTCCTCCAAGCTACTCTR: CTTGACGTTCTTCAGGTCTGG | NM_001310634.1 |
| gfap | F: AACCGCATCACCATTCCTGTR: ACCTCACCATCCCGCATCT | NM_010277.3 |
| mbp | F: CTATAAATCGGCTCACAAGGR: AGGCGGTTATATTAAGAAGC | NM_001025258.2 |
| sox2 | F: AAGGGTTCTTGCTGGGTTTTR: AGACCACGAAAACGGTCTTG | NM_011443.4 |
Statistical analysis
Data in this study was presented as mean ± SEM and analyzed by GraphPad Prism 6.0 software. Differences amongst treated groups were analyzed by two-way ANOVA followed by post-hoc Tukey’s multiple comparisons methods. Differences would be considered statistically significant when p-value ≤ 0.05.
Results
Spheroid bodies emerging from floating cells expressed neural stem cells markers
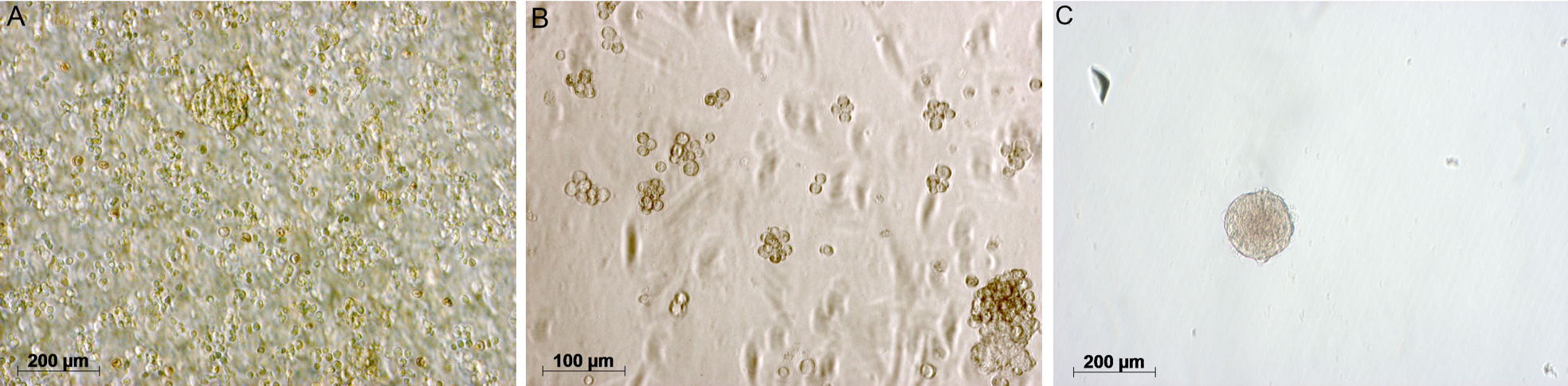
Three days since seeding, round-shape clumps of cell were seen in the culture (Figure 1A,B)23. Sphere formation assay showed that cells when seperated from the sphere could form new ones (Figure 1B,C). Cells inside spheres were Nestin-positive for neural stem/progenitor marker (Figure 2) and CD24-positive by flow cytometry analysis (Figure 3). As withdrawing EGF and bFGF as well as adding fetal bovine serum to the basal NSC medium, cells adhering upon the surface were GFAP-positive (Figure 4).
High concentration of n-butanol was non-neurotrophic, not sustaining the structure of cultured neurospheres
The n-butanol fraction 500 µg/mL was unable to maintain the integrity of cultured neurospheres (Figure 5), characterized with scattered cells and dark borders. However, ethanol and aqueous fractions at concentrations did not cause any significant neurosphere deformity. Low concentrations (50, 100, 200 µg/mL) of the n-butanol fraction seemed not toxic for the neurospheres.
| Fractions | Concentration(µg/mL) | Neurotrophic |
| n-butanol | 500 | - |
| 50 – 200 | + | |
| Aqueous | 50 – 500 | + |
| Ethanol | 50 – 500 | + |
Panax vietnamensis ethanol fraction 200 μg/mL could maintain the growth rate of treated neurospheres
At day 4, the basal NSC medium (control) sphere diameter reached the final enlargement of 17±4%. At day 4 n-butanol 200 μg/mL and aqueous fraction 200 or 500 μg/mL significantly increased the sphere diameter compared to the control: n-butanol – 30.7.4±4.23% (p≤0.001), aqueous fraction 200 μg/mL – 23.78±7.99% (p≤0.01), aqueous fraction 500 μg/mL – 22.98±7.99% (p≤0.01). However, there was no significant difference between the control and ethanol fraction 200 μg/mL neurosphere. At day 5, ethanol fraction 200 μg/mL increased the sphere diameter by approx. 28±3% (p≤0.001), and no noticeable deformity of treated spheres was seen (Figure 6). No difference was between the growth rate of basal NSC medium and ethanol/n-butanol fractions 50 or 100 μg/mL.
For the integrity in neurosphere structure, those treated with 200 μg/mL n-butanol fraction (Figure 7) or 500 μg/mL aqueous fraction (Figure 8) could not maintain the whole structure at the end of the experiment. These spheres were characterized with loose cells around the border, eventually adhering upon the surface. Interestingly, treated neurospheres had a high and stable rate of diameter increase in the first three days, and began to degrade afterward significantly.
Ethanol fraction of Panax vietnamensis 200 μg/mL elevated G2/M-phase cells and cell cycle-related genes
For further analysis on the gene expression and cell cycle, 200 μg/mL was chosen as the only concentration of each ginseng fraction. The G2/M percentage of the ethanol fraction neurospheres was 28.73±0.44% (p≤0.001) and aqueous fraction was 25.85±0.71% (p≤0.01). For comparison, the basal NSC medium had 16.88±2.76% G2/M. The proportion of G0/G1 phase declined in all fraction-treated groups compared with that of basal NSC medium (p≤0.0001); the most significant was that of n-butanol fraction-treated spheres (28.643± 1.63%, p≤0.0001). n-butanol fraction-treated spheres had 51.2±0.93% cells in S phase (p<0.001), but not significantly increase the proportion of G2/M-phase cells (20.20±0.71%) (Figure 9A).
Treating neurospheres with 200 μg/mL ethanol fraction significantly elevated the mRNA levels compared with those of the basal NSC medium: ki67 (4.605±6.48 fold-change), cycC (9.53±2.63 fold-change), cycD1 (22.47±8.18 fold-change), cycA1 (12.61±4.65 fold-change). However, there was a down-regulation in all surveyed genes compared with the basal NSC medium when treating spheres with the n-butanol or aqueous fraction (Figure 9B).
Maintaining high level of sox2 and gfap expression as treating neurospheres with Panax vietnamensis ethanol fraction at 200 μg/mL
To evaluate the differentiation effect of the ginseng fractions, neurospheres were cultured in EGF- and bFGF-free media, with the ginseng fraction added for five days. In addition, NGF (5 μg/mL) was also added as the positive control in the differentiation assay. In this study, there was a high mRNA level of sox2 (71.25±27.24 fold-change) and gfap (73.55±47.14 fold-change) as treating spheres with 200 μg/mL ethanol fraction. These levels were significantly different compared with those treated with the n-butanol fraction (sox2: 4.62±4.72 fold-change, p<0.05; gfap: 0.85±1.02 fold-change, p<0.01) and aqueous fraction (sox2: 5.77±1.44 fold-change, p<0.05; gfap: 0.66±0.20, p<0.05). The map2 mRNA level in ethanol fraction-treated neurospheres was up-regulated (4.605±3.33), but not statistically different from that in other groups. Interestingly, the mbp mRNA level of all treatment groups were down-regulated as compared with the negative control (Figure 10).
Discussion
In this study, cultured neural stem cells could persistently generate secondary spheres through 4 passages, and strongly expressed nestin and CD24, markers for neural lineage25,26. Neural stem/progenitor cells could differentiate to 3 distinct types in the neural lineage: neurons, astrocytes and oligodendrocytes 27. Cells from neurospheres could be induced to differentiate into the GFAP-positive astrocyte-like cells28, further confirming the expression of GFAP protein, which was previously mentioned by mRNA expression in our previous study23.
Our results show that ginseng extract fractions significantly promoted the neurosphere growth. Normally, quiescient cells predominantly present in cultured neurospheres29, which was confirmed by the high proportion of G0/G1 in those cultured with the basal NSC medium. When treating neurospheres with ginsenosides, it was shown that they promote the growth rate of neurospheres both in vitro and in vivo30,31. In this study, the P. vietnamensis ethanol fraction particularly enhanced the proliferation of neural stem cells compared with other fractions. Interestingly, there was a similar pattern between ethanol fraction- and NGF-treated neurospheres: up-regulated mRNA levels of proliferating genes and high G2/M proportion. In the presence of EGF and bFGF, nerve-growth factor (NGF) increases the number of nestin+ cells and promotes the survival and proliferation of neural stem cells32,33. Ginsenosides were shown to enhance the expression of the neurotrophic receptor such as p75, p21, TrkA in Neuro-2a cells 34 as well as elevate NGF and BDGF levels in cultured Schwann cells35. This suggest that the ethanol fraction might have similar effects of NGF on proliferating neurospheres.
In the differentiation assay, there was also a similar pattern between ethanol fraction- and NGF-treated neurospheres. Interestingly, our results indicated up-regulation of cycD1 mRNA and decrease in G0/G1 population effect in the proliferation assay (shown above), which suggests neurogenesis inhibition while self-renewal promotion36. This was correlated with the high sox2 mRNA level in the absence of EGF and bFGF coming from actively self-renewal cells37. In addition, actively proliferating neurospheres would contain GFAP+ core due to being partly isolated from mitogens38,39, correlating with the high mRNA level of gfap when eliminating EGF and bFGF from the medium. In this study, the ethanol fraction-treated neurospheres were more condensed than those with n-buthanol fraction indicating an increase in the size of individual cells rather than the cell number. This was consistent with a significantly high level of S-phase cells but low level of ki67 and cycC mRNA in n-butanol fraction-treated neurospheres40. As treating neurospheres with the aqueous fraction, low cycC mRNA level and S-phase proportion suggest that treated cell poorly entered active stages. With the presence EGF and bFGF in culture media, it’s noteworthy that the ginseng n-buthanol or aqueous fraction might have inhibiting effect on the neural stem cell proliferation.
Previous studies on Panax vietnamensis extract already presented its new ginsenosides and other bioactive substances 10,41 as well as its in vivo effects on the nervous system22. Others already pointed out positive effects of Panax ginseng extract/ginsenosides on nervous system in vivo of increasing SOX2 expression and promoting hippocampal proliferation14,42, attenuating neural stem cell scenescence43, maintaining neural stem cell proliferation in lead poisoning44. Because neural stem/progenitor cells still reside in the body, many questions concerning specific mechanisms of ginseng extract/ginsenosides still remain. Using an in vitro model of neurosphere, for the first time this study has provided new insights into proliferative and differentiative effects of the ginseng extract fractions, particulaly the ethanol fraction on the neural stem cell. However, further experiments should focus into specific Panax vietnamensis ginsenosides to elucidate how the ginsenosides could promote or inhibit the neural stem cell proliferation/differentiation.
Conclusions
In this study, Panax vietnamensis extract fractions of at specific concentrations had a positive effect on the proliferation of cultured neural stem cells. The ethanol fraction at 200 μg/mL could significantly promote the growth rate while still sustained the integrity of treated spheres. Treated neurospheres had high levels of cell cycle mRNA expression, high proportion of the G2/M cells, as well as the percentage of G0/G1 significantly decreased. Moreover, the fraction might have similar effects as those of NGF on the differentiation of neural stem/progenitor cells. Further study should be done to elucidate the mechanism in which each ginsenoside has its effects on neural stem cells.
Abbreviations
bFGF: basic fibroblast growth factor
EGF: Epidermal growth factor
GFAP: Glial Fibrillary Acidic Protein
NGF: Nerve growth factor
NSC: Neural stem cell
Competing Interests
The authors declare that they have no conflicts of interest.
Authors' Contributions
HQ Do and NH Truong carried out studies including gene-expression, flow cytometry, data analysis and manuscript composing. TT Lam, LT Nguyen, NHT Dinh and PTB Le isolated/cultured neural stem cells and tested gingseng fraction on neural spheres. LC Tran performed plant fractions for the experiment. NK Phan and PV Pham, who advised and orient the study, revised the manuscript, edited figures and checked the published data. All authors read and approved the final manuscript.
Acknowledgment
This study was funded by Department of Science and Technology, Ho Chi Minh city.
References
-
Kampen
J.M. Van,
Baranowski
D.B.,
Shaw
C.A.,
Kay
D.G.,
Panax ginseng is neuroprotective in a novel progressive model of Parkinson's disease. Exp Gerontol.
2014;
50
:
95-105
.
View Article PubMed Google Scholar -
Lee
S.T.,
Chu
K.,
Sim
J.Y.,
Heo
J.H.,
Kim
M.,
Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord.
2008;
22
(3)
:
222-6
.
View Article PubMed Google Scholar -
Ji
Y.C.,
Kim
Y.B.,
Park
S.W.,
Hwang
S.N.,
Min
B.K.,
Hong
H.J.,
Neuroprotective effect of ginseng total saponins in experimental traumatic brain injury. J Korean Med Sci.
2005;
20
(2)
:
291-6
.
View Article PubMed Google Scholar -
Shi
Y.Q.,
Huang
T.W.,
Chen
L.M.,
Pan
X.D.,
Zhang
J.,
Zhu
Y.G.,
Ginsenoside Rg1 attenuates amyloid-beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis.
2010;
19
(3)
:
977-89
.
View Article PubMed Google Scholar -
Liang
W.,
Ge
S.,
Yang
L.,
Yang
M.,
Ye
Z.,
Yan
M.,
Ginsenosides Rb1 and Rg1 promote proliferation and expression of neurotrophic factors in primary Schwann cell cultures. Brain Res.
2010;
1357
:
19-25
.
View Article PubMed Google Scholar -
Zheng
F.,
Wang
H.,
NMDA-mediated and self-induced bdnf exon IV transcriptions are differentially regulated in cultured cortical neurons. Neurochem Int.
2009;
54
(5-6)
:
385-92
.
View Article PubMed Google Scholar -
Li
Y.B.,
Wang
Y.,
Tang
J.P.,
Chen
D.,
Wang
S.L.,
Neuroprotective effects of ginsenoside Rg1-induced neural stem cell transplantation on hypoxic-ischemic encephalopathy. Neural Regen Res.
2015;
10
(5)
:
753-9
.
View Article PubMed Google Scholar -
Xu
F.T.,
Li
H.M.,
Yin
Q.S.,
Cui
S.E.,
Liu
D.L.,
Nan
H.,
Effect of ginsenoside Rg1 on proliferation and neural phenotype differentiation of human adipose-derived stem cells in vitro. Can J Physiol Pharmacol.
2014;
92
(6)
:
467-75
.
View Article PubMed Google Scholar -
Duong
Q.H.T.,
Nguyen
P. T. V.,
Nguyen
H. T. T.,
Nguyen
D. M.,
Effects of ocotillol-type saponins majonoside-R1 and vina-ginsenoside-R2 on abrogating depression and neuronal oxidative stress in socially isolated depression mouse model. International Journal of Applied Research in Natural Products.
2016;
9
:
27-32
.
-
Yamasaki
K.,
Bioactive saponins in vietnamese ginseng, panax vietnamensis. Pharm Biol.
2000;
38
(sup1)
:
16-24
.
View Article PubMed Google Scholar -
Huong
N.T.,
Murakami
Y.,
Tohda
M.,
Watanabe
H.,
Matsumoto
K.,
Social isolation stress-induced oxidative damage in mouse brain and its modulation by majonoside-R2, a Vietnamese ginseng saponin. Biol Pharm Bull.
2005;
28
(8)
:
1389-93
.
View Article PubMed Google Scholar -
Nguyen
M.D.,
Nguyen
T.N.,
Kasai
R.,
Ito
A.,
Yamasaki
K.,
Tanaka
O.,
Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. I. Chem Pharm Bull (Tokyo).
1993;
41
(11)
:
2010-4
.
View Article PubMed Google Scholar -
Peña
I.J. Dela,
Kim
H.J.,
Botanas
C.J.,
Peña
J.B. de la,
Le
T.H. Van,
Nguyen
M.D.,
The psychopharmacological activities of Vietnamese ginseng in mice: characterization of its psychomotor, sedative-hypnotic, antistress, anxiolytic, and cognitive effects. J Ginseng Res.
2017;
41
(2)
:
201-8
.
View Article PubMed Google Scholar -
Zhu
J.,
Mu
X.,
Zeng
J.,
Xu
C.,
Liu
J.,
Zhang
M.,
Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS One.
2014;
9
(6)
:
e101291
.
View Article PubMed Google Scholar -
Davis
S.F.,
Hood
J.,
Thomas
A.,
Bunnell
B.A.,
Isolation of adult rhesus neural stem and progenitor cells and differentiation into immature oligodendrocytes. Stem Cells Dev.
2006;
15
(2)
:
191-9
.
View Article PubMed Google Scholar -
Vishwakarma
S.K.,
Bardia
A.,
Tiwari
S.K.,
Paspala
S.A.,
Khan
A.A.,
Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J Adv Res.
2014;
5
(3)
:
277-94
.
View Article PubMed Google Scholar -
Rietze
R.L.,
Reynolds
B.A.,
Neural stem cell isolation and characterization. Methods Enzymol.
2006;
419
:
3-23
.
View Article PubMed Google Scholar -
Fang
Y.,
Eglen
R.M.,
Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS discovery: advancing life sciences R & D.
2017;
22
(5)
:
456-472
.
PubMed Google Scholar -
Ko
K.R.,
Frampton
J.P.,
Developments in 3D neural cell culture models: the future of neurotherapeutics testing?. Expert Rev Neurother.
2016;
16
(7)
:
739-41
.
View Article PubMed Google Scholar -
Reynolds
B.A.,
Weiss
S.,
Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science.
1992;
255
(5052)
:
1707-10
.
View Article Google Scholar -
Ferrari
D.,
Binda
E.,
Filippis
L. De,
Vescovi
A.L.,
Isolation of neural stem cells from neural tissues using the neurosphere technique. Curr Protoc Stem Cell Biol..
2010;
Chapter 2
(Unit2D.6)
.
View Article Google Scholar -
Nguyen
T.T.,
Matsumoto
K.,
Yamasaki
K.,
Nguyen
M.D.,
Nguyen
T.N.,
Watanabe
H.,
Crude saponin extracted from Vietnamese ginseng and its major constituent majonoside-R2 attenuate the psychological stress- and foot-shock stress-induced antinociception in mice. Pharmacol Biochem Behav.
1995;
52
(2)
:
427-32
.
View Article PubMed Google Scholar -
Nhung
H.T.,
Dinh
N. T. H.,
Le
D. M.,
Nguyen
L. T.,
Lam
T. T.,
Phan
N. K.,
Pham
P. V,
Isolation and culture of neural stem cells from murine foetal brain. Res. Opin. Anim. Vet. Sci..
2014;
4
(1)
:
24-29
.
-
Zheng
X.S.,
Yang
X.F.,
Liu
W.G.,
Shen
G.,
Pan
D.S.,
Luo
M.,
A novel method for culturing neural stem cells. In Vitro Cell Dev Biol Anim.
2007;
43
(5-6)
:
155-8
.
View Article PubMed Google Scholar -
Pruszak
J.,
Ludwig
W.,
Blak
A.,
Alavian
K.,
Isacson
O.,
CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells.
2009;
27
(12)
:
2928-40
.
View Article PubMed Google Scholar -
Ernst
C.,
Christie
B.R.,
The putative neural stem cell marker, nestin, is expressed in heterogeneous cell types in the adult rat neocortex. Neuroscience.
2006;
138
(1)
:
183-8
.
View Article PubMed Google Scholar -
Gage
F.H.,
Mammalian neural stem cells. Science.
2000;
287
(5457)
:
1433-8
.
View Article PubMed Google Scholar -
Bernal
A.,
Arranz
L.,
Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci.
2018;
75
(12)
:
2177-95
.
View Article PubMed Google Scholar -
Hulspas
R.,
Quesenberry
P.J.,
Characterization of neurosphere cell phenotypes by flow cytometry. Cytometry.
2000;
40
(3)
:
245-50
.
View Article PubMed Google Scholar -
Shen
L.H.,
Zhang
J.T.,
Ginsenoside Rg1 promotes proliferation of hippocampal progenitor cells. Neurol Res.
2004;
26
(4)
:
422-8
.
View Article PubMed Google Scholar -
Lin
T.,
Liu
Y.,
Shi
M.,
Liu
X.,
Li
L.,
Liu
Y.,
Promotive effect of ginsenoside Rd on proliferation of neural stem cells in vivo and in vitro. J Ethnopharmacol.
2012;
142
(3)
:
754-61
.
View Article PubMed Google Scholar -
Cattaneo
E.,
McKay
R.,
Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature.
1990;
347
(6295)
:
762-5
.
View Article PubMed Google Scholar -
Lachyankar
M.B.,
Condon
P.J.,
Quesenberry
P.J.,
Litofsky
N.S.,
Recht
L.D.,
Ross
A.H.,
Embryonic precursor cells that express Trk receptors: induction of different cell fates by NGF, BDNF, NT-3, and CNTF. Exp Neurol.
1997;
144
(2)
:
350-60
.
View Article PubMed Google Scholar -
Kim
M.S.,
Yu
J.M.,
Kim
H.J.,
Kim
H.B.,
Kim
S.T.,
Jang
S.K.,
Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol Pharm Bull.
2014;
37
(5)
:
826-33
.
View Article PubMed Google Scholar -
Liang
W.,
Ge
S.,
Yang
L.,
Yang
M.,
Ye
Z.,
Yan
M.,
Ginsenosides Rb1 and Rg1 promote proliferation and expression of neurotrophic factors in primary Schwann cell cultures. Brain Res.
2010;
1357
:
19-25
.
View Article PubMed Google Scholar -
Lange
C.,
Huttner
W.B.,
Calegari
F.,
Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell.
2009;
5
(3)
:
320-31
.
View Article PubMed Google Scholar -
Graham
V.,
Khudyakov
J.,
Ellis
P.,
Pevny
L.,
SOX2 functions to maintain neural progenitor identity. Neuron.
2003;
39
(5)
:
749-65
.
View Article PubMed Google Scholar -
Campos
L.S.,
Leone
D.P.,
Relvas
J.B.,
Brakebusch
C.,
Fässler
R.,
Suter
U.,
Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development.
2004;
131
(14)
:
3433-44
.
View Article PubMed Google Scholar -
Campos
L.S.,
Neurospheres: insights into neural stem cell biology. J Neurosci Res.
2004;
78
(6)
:
761-9
.
View Article PubMed Google Scholar -
Ren
S.,
Rollins
B.J.J.C.,
Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell.
2004;
117
(2)
:
239-51
.
View Article PubMed Google Scholar -
Tran
Q.L.,
Adnyana
I.K.,
Tezuka
Y.,
Nagaoka
T.,
Tran
Q.K.,
Kadota
S.,
Triterpene saponins from Vietnamese ginseng (Panax vietnamensis) and their hepatocytoprotective activity. J Nat Prod.
2001;
64
(4)
:
456-61
.
View Article PubMed Google Scholar -
Shen
L.H.,
Zhang
J.T.,
Ginsenoside Rg1 promotes proliferation of hippocampal progenitor cells. Neurol Res.
2004;
26
(4)
:
422-8
.
View Article PubMed Google Scholar -
Chen
L.,
Yao
H.,
Chen
X.,
Wang
Z.,
Xiang
Y.,
Xia
J.,
Ginsenoside Rg1 Decreases Oxidative Stress and Down-Regulates Akt/mTOR Signalling to Attenuate Cognitive Impairment in Mice and Senescence of Neural Stem Cells Induced by D-Galactose. Neurochem Res.
2018;
43
(2)
:
430-40
.
View Article PubMed Google Scholar -
Wang
B.,
Feng
G.,
Tang
C.,
Wang
L.,
Cheng
H.,
Zhang
Y.,
Ginsenoside Rd maintains adult neural stem cell proliferation during lead-impaired neurogenesis. Neurol Sci.
2013;
34
(7)
:
1181-8
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 10 (2019)
Page No.: 3422-3432
Published on: 2019-10-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 9094 times
- Download PDF downloaded - 1695 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress