Abstract
Overweight and obesity is currently a growing burden, according several research data from National Health and Morbidity Survey (NHMS) in Malaysia, shows that in 1999 overweight were at 16,6%, obesity 4.5%; in 2006, overweight were at 29.1%, obesity 14%, in 2011 overweight were, 29.4%, obesity 15.1% and in 2015 overweight were 30%, obesity 17.7%. Suggesting that there is parallel prevalence increase, in this sedentary life style in 2015 compared to 1999. Overweight and obesity affects multiple factor such as shifting lifestyles, urbanization, rising income and influence the genetic make-up of life. Currently, it is a great concerned in Malaysia, because it is a risk factor for most inflammatory disease such as cardiovascular disease, cancer and type 2 diabetes, stroke and chronic inflammatory disease, Recent information by Malaysian health minister, won that the country is facing overweight and obesity pandemic problem showing that half of Malaysian population were either overweight or obese. In addition, the latest estimates from the World Health Organization (WHO), show that almost 14% of the country's citizens fall under the ''obese'' category in Malaysia. A further 40% are overweight. Evidence has shown that PMPs interact with sub-endothelial matrix to mobilise monocytes and neutrophils that favour foam cells formation. PMPs play an important role in the transport and delivery of bioactive molecules that can signal inflammation and promote aberrant angiogenesis in diseases such as atherosclerosis, diabetic retinopathy and cancers in overweight. PMPs may affect target cells either by stimulating them via surface-expressed ligands or by transferring surface receptors from one cell to the others. The modulation effect of omega-3 fatty acid (EPA/DHA) from salmon on platelets and endothelial cell markers has been established. American Heart Foundation in collaboration with Committee on Evaluation, Prevention and Detection recommends 250 to 300 mg of cooked salmon per day for the treatment of high blood pressure and cardiovascular diseases. Yellow stripe scad (YSS) is a local Malaysian fish, recently identified to increase HDL-C in overweight subjects. However, the atheroprotective effect of omega-3 fatty acid from YSS on PMPs markers in overweight and obese healthy individuals is still unclear. Thus, the aim of this article is to explore the nutritional value (EPA/DHA) of YSS Fish fillet on platelet microparticles markers that predetermine overweight and obesity risk factors of atherosclerosis.
Introduction
Overweight and obesity in Malaysia
Overweight/obesity is a growing concern in Malaysia as diseases such as type 2 diabetes, cancer, cardiovascular disease, stroke and chronic diseases are reaching worrying levels, however, a well-balanced diet has vital roles in maintaining normal weight, promoting an overall healthy life and preventing chronic inflammatory disorders such as atherosclerosis, heart diseases, cancers and post-operative morbidity1. World Health Organization (WHO) in 2016 has shown that over 1.9 billion adults aged ≥ 18 years were overweight, whereas more than 650 million aged ≥ 18 years were obese. Recently, Malaysia was ranked as the country with second highest overweight population in South East Asia2,3. Current data from National Health and Morbidity Survey have shown that the prevalence rate of overweight male and female in Malaysia between 2011 and 2015 were at 29.4% and 30.0%, respectively, and the obesity prevalence rate were at 15.1% and 17.7%, respectively4,5,6,7. The presence of activated platelets in overweight subjects generates complex reactions that put healthy subjects at risk of pro-thrombotic state8. Atherosclerosis is an inflammatory disease characterized by abnormal lipid metabolism and storage disorder, that cause the infiltration of neutrophil, monocytes and macrophages into the endothelial matrix, via platelet activation or PMPs markers on the vascular arterial wall, for foam cell formation8. Foam cells are lipid rich core of fat laden, which are formed as a result of monocytes recruitment into the endothelial matrix in the earlier stage of atherosclerotic formation. Several studies have shown that omega-3 fatty acid (EPA+DHA) inhibits expression of platelet activation, PMPs and their related makers, leucocyte recruitment and foam cell formation9,10,11,12. Salmon fish fillet is well- known for its cardio-protective properties13,14,15. This review article highlights the complex interactions between overweight with platelets activation, PMPs and its related makers on endothelial cells and vascular smooth muscle cells (VSMC) for the pathogenesis of atherosclerosis. The dietary values of local Malaysian fish, the YSS (EPA/DHA) in comparison to foreign fish, the salmon (EPA/DHA) on PMPs markers to prevent atherosclerosis will also be discussed.
Association between Overweight, Leptin and Platelet Activation
Overweight is a low grade chronic inflammatory disorder that has been linked to chronic degenerative disease. Several literatures associated this metabolic disorder to excessive adipose tissue that is commonly accompanied by hypertriglyceridemia, hyperinsulinemia, insulin-resistance, decreased HDL cholesterol and hypertension. The occurrence of these multiple factors triggers the atherosclerotic lesions in the endothelium16. However, another possible mechanism is involved, i.e. haemostatic imbalance, particularly platelet hyper-reactivity, which aggravates the pathogenesis of atherosclerosis17. The latter hypothesis elicits the search for specific circulating factors that may be responsible for the enhanced amplification of acute thrombotic reciprocation to vascular endothelial bed injury in overweight and obese individuals.
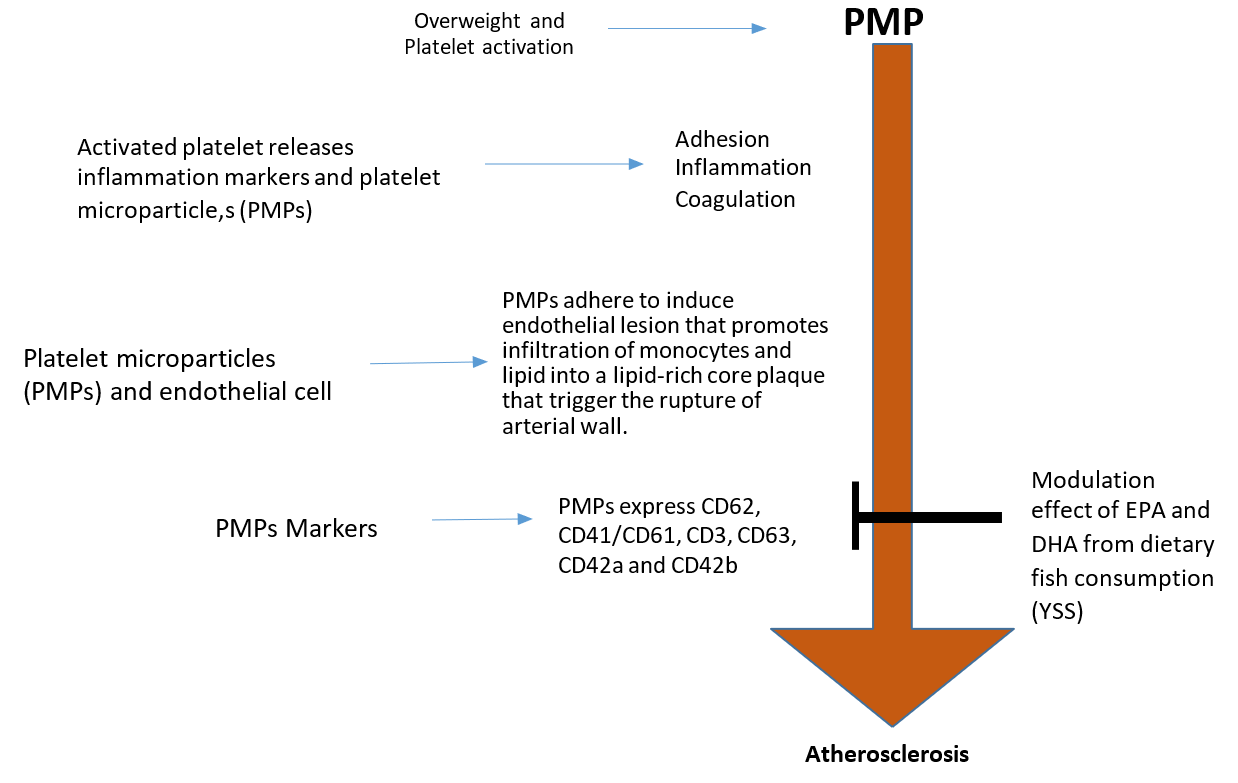
Overweight and obesity can be characterised as leptin resistance18. Leptin is an adiponectin hormone produced by OB gene and consists of 167-amino acids, functioning as fat and energy storage regulator in mammals18,19. Leptin acts directly in hypothalamic receptors to reduce food appetite and to enhance the energy expenditure level by regulating both fatty acid and glucose metabolism in normal subjects18. In fact, studies have shown that high concentration of leptin demonstrates signs of leptin resistance19, and the ob/ob mouse that lacks functional leptin, due to nonsense mutation in codon 105 ob — gene, develops severe hyperplasia and excessive obesity20. Thus, leptin plays a significant role in this diet-related disorder. Previous literatures also reported that leptin has complex biological roles more/less than the body weight modulation; i.e. leptin was reported to regulate angiogenesis, immune function, fertility, and bone formation, and the presence of leptin receptors can be found in various types of tissues21,22,23. Human platelets were shown to possess the long form of leptin receptors24 and high levels of leptin were shown to act collegially along with ADP to foster platelet hype-reaction in-vitro25. These observations have shown the possibility that leptin resistance may contribute to atherosclerotic lesion that alters the haemostatic balance in overweight subjects (Figure 1).
Activated platelets release inflammation markers and platelet microparticles (PMPs)
Activated platelets release multiple platelet activation markers, which are associated with vascular inflammation and thrombosis that include soluble CD40 ligand (sCD40L), P-selectin, Regulated on Activation, Normal T-cell Expressed and Secreted (RANTES), transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), thromboxane-A2 (TXA2) and platelet factor 4 (PF-4). RANTES mobilises T-cells and monocytes to the injured endothelial cells/VSMC via platelet P-selectin to stimulate monocyte migration and arrest into the vascular endothelium26,27. PDGF is a platelet growth factor that plays significant roles in proliferation, migration and stimulation of VSMC in the presence of serotonin and TGF- β27,28. PDGF also acts as monocyte and neutrophil chemotactic factor and signalling via the platelet-derived growth factor receptor (PDGFR) pathway. This pathway plays a significant role in VSMC migration and proliferation, which is a primary characteristic of atherosclerosis29,30,31. PF-4 is a subfamily of CXC chemokine that promotes the differentiation of monocytes into foam cell formation through the interaction of oxidized low-density lipoproteins (LDL) and injured endothelial cells. It also protects the oxidized LDL receptors from degradation32,33. sCD40L is a soluble protein released from alpha granules of activated platelets with the structural resemblance to TNF-α superfamily, which is found on the exposed surface of activated platelets. sCD40L increases the stability of new platelet aggregation and facilitates the inflammatory mechanism that involves vascular endothelial cells and reactive oxygen species (ROS). This leads to the expression of adhesion molecules such as Intercellular Adhesion Molecule-1 (ICAM-1), E-selectin and Vascular Cell Adhesion Molecule-1 (VCAM-1) in both VSMC and endothelial cells, including the secretion of matrix metalloproteinase (MMPs), procoagulant factors, cytokines and chemokines34,35. Activated platelets also release PMPs for their normal physiological function. The small size and structure of PMPs make them an important cargo in platelet cell communication and a delivery agent of platelet — borne bio-active molecules such as platelet activation markers, platelet inflammation cytokines, signalling molecules, growth factors, and microRNAs (miRNA)36. An increased level of plasma PMPs was shown to occur in subjects with few pathological conditions such as overweight with type 2 diabetes or non-overweight with type 2 diabetes37. In contrast, overweight subjects in the absence of diabetic condition shows no effect of PMPs markers, but an increased level of PMPs without markers was observed37. Increased levels of specific positive PMPs markers in subjects with a diabetic condition may provide a potential pathway by which PMPs contribute to the pathogenesis of atherosclerosis and cardiovascular events Figure 2.
Janowska-Wieczorek and colleagues have shown that haematopoietic stem-progenitor cells expressed PMPs membrane receptors such as CD62, CD41, PAR-1 and CXCR4 that encourage the engraftment of progenitor cells to the endothelium38. This observation suggests that PMPs from activated platelets play a significant role in stem cell transplantation and recipient cells, which affects the cell function of the recipients. PMPs engulf cytoplasmic membrane during their formation to acquire protein and RNA from the cytosol of the parent cells. Multiple evidence have suggested that following the attachment or fusion of PMPs to the target cells, PMPs will transfer the RNA and protein content to the recipient cells39,40,41. This process facilitates the recipient cell to internalise PMPs via endocytosis or through receptor-ligand interactions42, which suggests that PMPs could reprogram a target cells. Ratajczak et al., shown that microparticles derived from embryonic stem cells reprogramed the haematopoietic stem cell progenitors via the horizontal transfer of protein and mRNA delivery43. Other studies have shown that microparticles from endothelial cell induced angiogenesis in both in-vivo and in-vitro via horizontal transfer of mRNA to human macrovascular and microvascular endothelium44. The CD41+ microparticles are known to have coagulant properties, however, the anticoagulant properties are still unknown.
Pathophysiology of PMPs and its related markers in atherosclerosis
PMPs are known to play an important haemostatic role, by which the inability of platelets to generate PMPs was linked to bleeding episode (Castaman's defect)45. Secondly, platelets in patient with Scott's syndrome shows impaired ability to generate PMPs and displays a bleeding diathesis. PMPs were demonstrated to play significant roles in the activation of vascular endothelial cells, exposure of PMPs to endothelial phospholipase A2, and stimulation of arachidonic acid release from both platelets and endothelial cells, which are subsequently metabolised to produce thromboxane A246. The trans-activation of both platelets and endothelial cells further promotes the interactions of monocyte endothelial cells47. Berckmans and colleagues reported that the presence of PMPs in healthy individuals, particularly the CD41+ micropartiples, are known to generate thrombin48. CD41+ microparticles are highly procoagulant in nature. The presence of CD41+ receptors on PMPs may contribute to the pathogenesis of arterial thrombosis disease. Several literatures have demonstrated that circulating PMPs may provide a potential diagnostic and prognostic makers of atherosclerotic vascular disease2,49,50,51. CD63-exposing and P-selectin from PMPs reflect the platelet activation markers in myocardial infarction and peripheral arterial disease52. Michelsen et al., have shown that increased levels of PMPs was observed in myocardial infarction survivors53. PMPs have been shown to be independent of large PMPs, soluble CD40 ligand (sCD40L) and plasma thrombin-antithrombin complexes in patients with myocardial infarction, but not in healthy controls. Another study by Chironi et al. demonstrated that the diameter of both internal and external carotid artery were negatively correlated with PMPs derived from platelets, endothelial cells and leucocytes54. It is, however, still unclear about the effect of omega-3 fatty acid (EPA/DHA) from YSS fish fillet on PMPs (CD41) and its related markers such as P-selectin (CD62P), CD42a (GpIb), GPIb-V-IX, CD63, platelet endothelium adhesion molecule-1 (PECAM-1(CD31), integrin glycoprotein (GP) IIb/IIIa.
Platelet microparticles (PMPs) in endothelial cells and atherosclerosis
Endothelial cells (ECs) are the barrier that maintains the balance between the circulatory molecules and the cells. It is the key modulator of vascular homeostasis, which plays an essential role in signal transduction that affects the reorganization of vascular wall physical composition55. ECs release certain vascular - balanced chemical molecules such as nitric oxide (NO), endothelia, prostaglandin I2 and endothelium-derived relaxing factor that maintains the integrity of vascular beds. It also releases anticoagulation proteins such as tissue factors and plasminogen activators, which are important in fibrinolytic system. Thus, healthy and normal ECs inhibit the adhesion and aggregation of platelets to vascular endothelial wall, which facilitates a range vascular tone regulatory factors including smooth muscle cell proliferation, vascular wall inflammation, cellular adhesion and thromboresistance56. The release PMPs from activated platelets systematically affects the activities of other cell types as summarized in Figure 3 57. PMPs are protein and lipid complexes with an average size of ≤ 1 µm. It is a minor fragment of platelet α-granules and phospholipid membrane58. Apart from other microparticles present in circulation, PMPs are made up of 70%–90% of circulatory blood and their cargo content are relevant in both inflammation and haemostasis, as it also enhance coagulation, mediate leucocytes adhesion to subendothelial matrix and promote stimulation of VSMC angiogenesis59,60. Thus, the effect of PMPs markers on endothelial cells and VSMC can be characterized as chronic inflammation61 and one of the observed effects of PMPs markers on endothelial cells is the synthesis of interleukin 1 beta (IL-1β). IL-1β is an active cytokine that stimulates the production of adhesion and chemoattractant molecules such as MCP-1, VCAM-1 and ICAM-1 via NF-k B61. Thus, PMPs can be characterised as active mediators between the leucocytes and inflammatory cytokines62. PMPs markers recruit monocytes and RANTES to the site of injured endothelial cells63. The quantification of PMPs and its related markers can be conducted using two methods, the flow cytometer measurement (FCM) and enzyme-linked immunosorbent assays (ELISA)64,3. PMPs can be distinguished from other exosomes obtained from multivesicular endosomes as smaller MPs are found in α-granule-derived proteins while larger MPs are derived from platelet phospholipid membrane that express mitochondrial proteins. Thus, the evaluation of PMPs markers such as P-selectin (CD62P), CD42a (GpIb) and GPIb-V-IX, CD63, platelet endothelium adhesion molecule-1 (PECAM-1(CD31), integrin glycoprotein (GP) IIb/IIIa, CD41 and CD6165,66 in overweight subjects can be obtained using flow cytometry and the therapeutic effect of omega-3 fatty acid (EPA/DHA) from YSS fish fillet on these markers will be monumental to treat these diet-related disorders (Figure 3).
Selected platelet microparticles (PMPs) marker in atherosclerosis
P-selectin (CD62P): is an adhesion molecule that links PMPs to endothelial cells (ECs). Increased levels of PMPs and P-selectin in blood circulation is a sign of diabetes, hyperlipidemia, hypertension and overweight. P-selectin in diabetes patients are known to be associated with thrombotic events, such as retinopathy, coronary heart disease and atherosclerosis. The PMPs level is associated with platelet activation by expression of platelet P-selectin67. It is indeed, to note that, PMPs target in cardiovascular treatment are still limited68. PMPs with omega-3 fatty from YSS may modulate P-selectin levels as compared to those from salmon in both platelet and ECs.
CD42a (GpIb) and GPIb-V-IX, or GPIbα/Ibβ/V/IX : are also known as CD42bα/CD42bβ/ CD42d/CD42a respectively, are four of the glycoproteins belong to leucine-rich family. It facilitates platelets adhesion to vWF during vascular endothelial damage69. Deficiency of one of these four proteins can lead to Bernard-Soulier syndrome, which is a severe blood bleeding disorder. The interaction between vWF and GPIb-V-IX70 signals the transduction to activate platelet integrin (GPIIb/IIIa). This mechanism was observed in Apoe-/- mice injected with GPIbα, by which a drastic reduction in atherosclerosis lesion size was observed71. The observation indicates that GPIbα binding to vWF on endothelial cell is highly pro-inflammatory.
PMPs and platelet integrin aIIb β3 (GpIIb-IIIa): Platelet integrin belongs to the fibrinogen receptor family that is crucial for platelet activation, which can be found on the platelet membrane and the CD41/CD61 complex. Platelet integrin is also known as glycoprotein (GP) IIb/IIIa. The combination of CD41 and CD61 is associated with non-covalent bonding with fibrinogen, which is the basis of platelet aggregation72. Thus, the presence of this receptor (IIb/IIIa) in PMPs during acute coronary disorder allows the binding of PMPs to vascular wall, which will initiate a signal transduction pathway such as extracellular signal-regulated kinase, phosphoinositide 3-kinase (PI3-kinase) pathways, and activation of pertussis-toxin-sensitive G protein73. Studies have shown the association between high levels of PMPs with various diseases such as sickle cell disease, malignant tumour formation, thrombocytopenic purpura, cardiovascular diseases and haemolytic disorders65,74.
Modulation effects of fish fillet (EPA and DHA) on platelet microparticles (PMPs) markers
A meta-analysis of randomised clinical intervention trials have shown that omega-3 fatty acid (EPA/DHA) reduced the procoagulant function of platelets and cardiovascular disease (CVD)75. Other studies have shown a significant beneficial effects of EPA/DHA on platelet functions in type 2 DM and coagulation profile in patients with vascular complications, with marginal favourable effects on the glycaemic status and lipid profiles76. A pilot study of omega-3 fatty acid supplementation have shown the occurrence of attenuated platelet activation even among patients that took aspirin or aspirin plus clopidogrel77. Omega-3 fatty acid (EPA/DHA) exerts a gender-based dependent effects on platelet microparticles and platelet activation but not on MP levels. With regards to thrombotic disease risk, males may benefit more from EPA supplementation78. Other effects of EPA/DHA include protection against CVD by reducing serum triacylglycerides (TAG)79, heart rate80, blood pressure (BP)81, and serum homocysteine levels82, as well as regulating and resolving inflammatory process83 as shown in randomized trials with fish oil supplementation. However, other reports have shown that omega-3 have no effect on platelet aggregation84. Despite so, there is a paucity of available data on yellow stripe scad (YSS) as a source of omega-3 fatty acid in overweight healthy subjects.
Current available data
In human, immune cells such as monocytes, lymphocytes, neutrophils and platelets are regarded as inflammatory cells that protect the body against foreign invaders. High levels of omega-3 fatty acid (EPA/DHA) in the phospholipid membrane allows the cells to function effectively. However, in overweight individuals, the level of EPA/DHA is compromised with the higher level of omega-6 fatty acid (Arachonic acid) at 10-20% that decreases docosahexaenoic acid (DHA) to 2–4% and eicosapentaenoic acid (EPA) to 0.5–1%85,86. Even so, the level of omega-3 fatty acid in phospholipid membrane can be altered as omega-3 fatty acid can be obtained from fish and plant sources, despite the fact that the body does not produce omega-3 fatty acid. Currently, three types of fish samples were identified to contain a desirable amount of omega-3 fatty acids (EPA/DHA). Yellow stripe scad (YSS) showed the highest DHA with a Polyunsaturated/Saturated (P/S) = 1.7, moonfish showed the highest ALA with a P/S = 1.0, and longtail shad showed the highest EPA with P/S = 0.4)87. The intake of omega-3 fatty acid from fish fillets, especially from salmon, was shown to change the ratio of omega-6 to omega-3 fatty acid in favour of omega-3 phospholipid membrane that modulates the cellular inflammation via blood cells in overweight and obese subjects. Previous study by A bdulazeez et al., 2013 has shown that omega-3 fatty acid content in most Malaysian fishes contain lower EPA that falls between 2.7–343.0 mg/100 g wet sample87 in comparison to salmon feed (2638 mg/100 g), farmed salmon (1079 mg/100 g), supermarket salmon (969 mg/100 g), and wild salmon (414 mg/100 g)88. However, longtail shad with 1327.4 mg/100 g wet sample EPA88 was found to be comparable to salmon feed (2638 mg/100 g wet sample),88 suggesting that EPA from longtail shad can be associated with protective effects from the occurrence of asthma, coronary heart problems and many other diseases89,90. Meanwhile, DHA values (629–2633 mg/100 g) from previous studies on salmon fish fillets have shown that it is not comparable to the values (9.0–277.1 mg/100 g wet sample) by Abdul-Aziz et al., 2013. However, YSS with 782.1 mg/100 g wet sample was found to contain slightly higher DHA content as compared to wild salmon (629 mg/100 g)88. A study by Chang WL et al., 2017 showed that YSS could increase HDL-C levels in overweight subjects91, further suggesting that YSS fish fillets are comparable to wild salmon, and the omega-3 fatty acid in salmon fish fillets was shown to be cardioprotective. American Heart Foundation recommends the intake of omega-3 fatty acid at 250–300 mg/day for heart disease patients and it is indeed, to note that majority of fish oil capsule production contains salmon. Despite so, the effect omega-3 fatty acid (EPA/DHA) from YSS on PMPs and thrombosis is still unclear. Therefore, it is promising that the consumption of YSS at 250-300 mg/day with reference to recommendation by American Heart Foundation92 may ameliorates overweight/obesity -related risk factors such as platelet activation, PMPs and cardiovascular diseases.
Conclusion
This review article emphasised the role of EPA/DHA in overweight and obesity subjects on PMPs- related biomarkers. The EPA/DHA content in fishes differs from one fish to another in terms of quantity and quality. Despite so, several studies have shown the comparisons between EPA/DHA content of salmon and other fatty fishes. DHA is quantitatively the most important omega-3 PUFA in salmon and has consistently been shown to have unique and indispensable roles in platelet phospholipid membrane and cardiovascular diseases. Overweight and obesity subjects were shown to have deficits in EPA/DHA levels due to the higher levels of omega-6 fatty acid (arachidonic acid) on the platelet cellular membrane. Therefore, it is therapeutically promising. Intervention studies in 2017 have shown that EPA and DHA from YSS increase d the levels of HDL-C in overweight subjects, suggesting that the consumption of YSS at 250-300 mg/day could reduce the levels of platelet activation, PMPs marker, atherosclerosis and other related risk factors in overweight healthy people.
Abbreviations
ADP: Adenosine diphosphate
BP: Blood Pressure
CD41/CD61: integrin glycol protein (GP) IIb/IIIa
CD41+: Platelet microparticle marker
CD42a: GpIb, GPIb-V-IX,
CD62: P-selectin
CD62E: E-selectin
CVD: Cardiovascular Disease
CXC: chemokine
CXCR4: CD184 chemokine
DHA: Docosahexaenoic Acid
ECs: Endothelial cells
ELISA: Enzyme-Linked Immunosorbent Assays
EPA: Eicosapentaenoic Acid
HDL-C: High-Density Lipoprotein Cholesterol
ICAM-1: Intercellular Adhesion Molecule-1
IL-1 β: Interleukin-1 Β
LDL-C: Low-density lipoprotein (LDL) cholesterol
MCP-1: Monocyte Chemoattractant Protein-1
miRNA: microRNAs
MMPs: Matrix metallopeptidases
NO: Nitric Oxide
OB-gene: Obesity gene
PAR-1: Protease-Activated Receptors-1
PDGF: Platelet-Derived Growth Factor
PDGFR: Platelet-Derived Growth Factor Receptor
PECAM: Platelet Endothelium Adhesion Molecule-1 (CD31),
PF-4: Platelet factor 4
PI3: Phosphatidylinositol3-kinase
PMPs: Platelet Micro-Particles
RANTES: Regulated on Activation Normal T-cell Expressed and Secreted
ROS: Reactive Oxygen Species
sCD40L: Soluble CD40 ligand
TAG: Triacylglycerides
TGF-β: Transforming growth factor-β
TNF-α: Tumor Necrosis Factor alpha
TXA2: Thromboxane-A2
VCAM-1: Vascular Cell Adhesion Molecule-1
VSMC: Vascular Smooth Muscle Cells
vWF: Von Willebrown Factor
WHO: Word Health Organization
YSS: Yellow Stripe Scad
Competing Interests
The authors declare no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Conception and design of study: Yakubu Abdulrahman, Azrina Azlan, Loh Su Peng, , Irmi Zarina Ismail Sabariah Md Noor (all authors)
Acquisition of data: Yakubu Abdulrahman
Analysis and/or interpretation of data: All authors
Drafting the manuscript: Yakubu Abdulrahman, Sabariah Md Noor
Revising the manuscript critically for important intellectual content: Azrina Azlan, Loh Su Peng, Irmi Zarina Ismail, Sabariah Md Noor
Approval of the version of the manuscript to be published (the names of all authors must be listed): Yakubu Abdulrahman, Azrina Azlan, Loh Su Peng, Irmi Zarina Ismail, Sabariah Md Noor
References
-
Kylasov
A,
Gavrov
S,
Diversity of Sport: non-destructive evaluation. UNESCO: Encyclopedia of Life Support Systems.
2011;
:
462-91
.
-
M Ng,
T Fleming,
M Robinson,
B Thomson,
N Graetz,
C Margono,
E C Mullany,
S Biryukov,
C Abbafati,
S F Abera,
J P Abraham,
Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet.
2014;
384
(9945)
:
766-81
.
View Article Google Scholar -
Flow Cytometry 2012.
View Article Google Scholar -
National Health And Morbidity Survey ( Nhms ) 2017 : Key Findings from the Adolescent Health and Nutrition Surveys. 2018;(April):1-29.
.
-
Tsioufis
K P,
Dimitriadis
K,
Koutra
E,
The metabolic syndrome: Requiescat in Pace. Clin Chem.
2005;
51
(6)
:
931-938
.
-
Institute for Public Health.
National Health and Morbidity Survey 2015 (NHMS 2015). Vol. II: Non-Communicable Diseases, Risk Factors & Other Health Problems. Vol II. 2015
.
View Article Google Scholar -
Ying
Y C,
Kuang
K L,
Kuang
H L,
Chien
H T,
Chee
C K,
Siew
M C,
Yi
Y K,
Physical activity and overweight/obesity among Malaysian adults: findings from the 2015 National Health and morbidity survey (NHMS). BMC Public Health.
2017;
17
:
733-733
.
View Article Google Scholar -
I Mertens,
LF
L F V Gaal,
Obesity , haemostasis and the fibrinolytic system. Obes Rev.
2002;
3
(2)
:
85-101
.
-
Domschke
G,
Gleissner
C A,
CXCL4-induced macrophages in human atherosclerosis. Cytokine.
2017;
(17)
:
30254-30259
.
View Article Google Scholar -
Amanda
L B,
Xuewei
Z,
Shunxing
R,
Swapnil
S,
Jeongmin
S,
.
Elena B,
Omega-3 Fatty Acids Ameliorate Atherosclerosis by Favorably Altering Monocyte Subsets and LimitingMonocyte Recruitment to Aortic Lesions. Arteriosclerosis, Thrombosis, and Vascular Biology.
2012;
32
:
2122-2130
.
View Article Google Scholar -
McEwen
B.J.,
The influence of diet and nutrients on platelet function. Semin Thromb Hemost.
2014;
40
(2)
:
214-240
.
View Article Google Scholar -
Turco
Del,
Basta
S,
Lazzerini
G,
Evangelista
G,
Rainaldi
M,
Tanganelli
G,
.
P,
Effect of the administration of n-3 polyunsaturated fatty acids on circulating levels of microparticles in patients with a previous myocardial infarction. Haematologica.
2008;
93
(6)
:
892-901
.
View Article Google Scholar -
Seierstad
S L,
Seljeflot
I,
Johansen
O,
Hansen
R,
Haugen
M,
Rosenlund
G,
Dietary intake of differently fed salmon; the influence on markers of human atherosclerosisEur. J Clin Invest.
2005;
35
(1)
:
52-61
.
View Article Google Scholar -
Parolini
C,
Vik
R,
Busnelli
M,
A salmon protein hydrolysate exerts lipid-independent anti-atherosclerotic activity in apoE-deficient mice. PLoS One.
2014;
9
(5)
:
1-9
.
View Article Google Scholar -
Vikren
L A,
Drotningsvik
A,
Bergseth
M T,
Mjs
S A,
Mola
N,
Leh
S,
Effects of baked and raw salmon fillet on lipids and n-3 PUFAs in serum and tissues in Zucker fa/fa rats. Food Nutr Res.
2017;
12
(1)
:
1333395-1333395
.
View Article Google Scholar -
J Kaur,
A comprehensive review on metabolic syndrome. Cardiol Res Pract.
2014;
:
943162-943162
.
View Article Google Scholar -
Jax
T W,
Peters
A J,
Plehn
G,
Schoebel
C,
Hemostatic risk factors in patients with coronary artery disease and type 2 diabetes - a two year follow-up of 243 patients. Cardiovasc Diabetol.
2009;
7
:
48-48
.
View Article Google Scholar -
Martin
G M,
Rudolph
L,
S
Randy J,
-,
Obesity and Leptin Resistance: Distinguishing Cause from Effect. Trends Endocrinol Metab.
2010;
21
(11)
:
643-651
.
View Article Google Scholar -
Harris
R B S,
Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta.
2014;
1842
(3)
:
414-437
.
View Article Google Scholar -
Lindstrom
P.,
The physiology of obese-hyperglycemic mice. Scientific World Journal.
200729;
7
:
666-685
.
View Article Google Scholar -
Fantuzzi
G,
Faggioni
R,
Leptin in the regulation of immunity , inflammation , and hematopoiesis. 2000;
68
:
437-446
.
View Article Google Scholar -
Elbatarny
H S,
Maurice
D H,
Leptin-mediated activation of human platelets: involvement of a leptin receptor and phosphodiesterase 3A-containing cellular signaling complex. Am J Physiol Endocrinol Metab.
2005;
289
(4)
:
695-702
.
View Article Google Scholar -
Priego
T,
Snchez
J,
Palou
-,
Pic
C,
Effect of high-fat diet feeding on leptin receptor expression in white adipose tissue in rats: depot- and sex-related differential response . Genes Nutr.
2009;
4
:
151-156
.
View Article Google Scholar -
Giandomenico
G,
Dellas
C,
Czekay
R P,
Koschnick
S,
Loskutoff
D J,
The leptin receptor system of human platelets. J Thromb Haemost.
2005;
3
(5)
:
1042-1051
.
View Article Google Scholar -
Lukasik
M,
Michalak
S,
Dworacki
G,
Siewiera
K,
Kaczmarek
M,
Watala
C,
Reactive leptin resistance and the profile of platelet activation in acute ischaemic stroke patients. Thromb Haemost.
2012;
108
(1)
:
107-125
.
View Article Google Scholar -
Vilahur
L,
.
G,
Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med.
2014;
276
(6)
:
618-650
.
View Article Google Scholar -
Sonmez
O,
Sonmez
M,
Role of platelets in immune system and inflammation. Porto Biomedical Journal.
2017;
2
(6)
.
-
Blair
P,
Flaumenhaft
R,
Platelet a -granules : Basic biology and clinical correlates. Blood Rev.
2009;
23
(4)
:
177-189
.
View Article Google Scholar -
Deuel
T. F.,
Senior
R. M.,
Huang
J. S.,
Griffin
G. L.,
Chemotaxis of Monocytes and Neutrophils to Platelet-derived Growth Factor. J Clin Invest.
1982;
69
(4)
:
1046-1055
.
View Article Google Scholar -
Martin
R,
Bennett
Sanjay,
Sinha
Gary K,
Owens
-,
Vascular smooth muscle cells in atherosclerosis. Circ Res.
2016;
118
:
692-702
.
View Article Google Scholar -
Montecucco
F,
Mach
F,
Atherosclerosis is an inflammatory disease. Semin Immunopathol.
2009;
31
(1)
.
View Article Google Scholar -
Lindemann
S,
Kra
B,
Seizer
P,
M
Gawaz,
-Platelets, inflammation and atherosclerosis. J Thromb Haemost.
2007;
5
.
View Article Google Scholar -
Aloui
C,
Prigent
A,
Sut
C,
Tariket
S,
Hamzeh-Cognasse
H,
Pozzetto
B,
The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci.
2014;
3
(12)
:
22342-64
.
View Article Google Scholar -
Kooten
C. Van,
Banchereau
J.,
CD40-CD40 ligand. J Leukoc Biol.
2000;
67
(1)
:
2-17
.
View Article Google Scholar -
Prasad
Kss,
Andre
P,
He
M,
Bao
M,
Manganello
J,
Phillips
D R,
Soluble CD40 ligand induces 3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci.
2003;
100
(21)
:
12367-12374
.
-
Lazar
S,
Goldfinger
L E,
Platelet Microparticles and miRNA Transfer in Cancer Progression: Many Targets, Modes of Action, and Effects Across Cancer Stages. Front Cardiovasc Med..
2018;
28
(5)
:
13
.
View Article Google Scholar -
Stepanian
A,
Bourguignat
L,
Hennou
S,
Coupaye
M,
Hajage
D,
Salomon
L,
Microparticle Increase in Severe Obesity : Not Related to Metabolic Syndrome and Unchanged after Massive Weight Loss. 2013;
21
(11)
:
2236-2243
.
View Article Google Scholar -
Janowska-Wieczorek
A,
Majka
M,
Kijowski
J,
Reca
R,
Turner
A R,
Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood.
2001;
98
(10)
:
3143-3149
.
View Article Google Scholar -
Ratajczak
J,
Wysoczynski
M,
Hayek
F,
Janowska-Wieczorek
A,
Ratajczak
M Z,
Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia.
2006;
20
(9)
:
1487-1495
.
View Article Google Scholar -
Laffont
B,
Provost
P,
Activated platelets can deliver mRNA regulatory Ago2 microRNA complexes to endothelial cells via microparticles. Blood.
2013;
122
(2)
:
253-61
.
View Article Google Scholar -
Xia
L,
Zeng
Z,
Tang
W H,
The Role of Platelet Microparticle Associated microRNAs in Cellular Crosstalk. Front Cardiovasc Med.
2018;
5
:
29-29
.
View Article Google Scholar -
Clayton
A,
Turkes
A,
Dewitt
S,
Steadman
R,
Mason
M D,
Hallett
M B,
Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J.
2004;
18
(9)
:
977-986
.
View Article Google Scholar -
Ratajczak
J,
Miekus
K,
Kucia
M,
Zhang
J,
Reca
R,
Dvorak
P,
Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia.
2006;
20
(5)
:
847-856
.
View Article Google Scholar -
Deregibus
M C,
Cantaluppi
V,
Calogero
R,
Tetta
C,
Biancone
L,
Endothelial progenitor cell - Derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood.
2007;
110
(7)
:
2440-2448
.
View Article Google Scholar -
Castaman
G,
Yu-Feng
L,
Rodeghiero
F,
A bleeding disorder characterised by isolated deficiency of platelet microvesicle generation. Lancet.
1996;
347
(9002)
:
700-701
.
View Article Google Scholar -
O P Barry,
Pratic
D,
Lawson
J A,
Fitzgerald
G A,
Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest.
1997;
99
(9)
:
2118-2127
.
View Article Google Scholar -
O P Barry,
Pratic
D,
Savani
R C,
Fitzgerald
G A,
Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest.
1998;
102
(1)
:
136-144
.
View Article Google Scholar -
Hack
C E,
Bing
A N,
Sturk
A,
Romijn
F P,
Berckmans
R J,
Nieuwland
R,
Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost.
2001;
85
(4)
:
639-646
.
-
Boulanger
C M,
Amabile
N,
Tedgui
A,
Circulating microparticles: A potential prognostic marker for atherosclerotic vascular disease. Hypertension.
2006;
48
(2)
:
180-186
.
View Article Google Scholar -
Wang
M,
Fu
Y,
Xu
Lan,
Yue
Yongjian,
Liu
Shengguo,
Diagnostic value of platelet ‑ derived microparticles in pulmonary thromboembolism : A population ‑ based study. Exp Ther Med.
2018;
16
(4)
:
3099-3106
.
View Article Google Scholar -
Yun
S,
Sim
E,
Goh
R,
Park
J,
Han
J,
Platelet Activation : The Mechanisms and Potential Biomarkers. Biomed Res Int.
2016;
:
9060143-9060143
.
View Article Google Scholar -
Van Der Zee
P M,
Bir
E,
Ko
Y,
De Winter
R J,
Hack
C E,
Sturk
A,
Nieuwland
R,
P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem.
2006;
52
(4)
:
657-664
.
View Article Google Scholar -
Michelsen
A E,
Brodin
E,
Brosstad
F,
Hansen
J B,
Increased level of platelet microparticles in survivors of myocardial infarction. Scand J Clin Lab Invest.
2008;
68
(5)
:
386-392
.
View Article Google Scholar -
Chironi
G N,
Simon
A,
Boulanger
C M,
Dignat-George
F,
Hugel
B,
Megnien
J L,
Circulating microparticles may influence early carotid artery remodeling. J Hypertens.
2010;
28
(4)
:
789-796
.
View Article Google Scholar -
Rodrigues
S F,
Granger
D N,
Blood cells and endothelial barrier function. Tissue Barriers.
2015;
3
(1-2)
:
978720-978720
.
View Article Google Scholar -
Bauer
V,
Sotnkov
R,
Nitric oxide - the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys.
2010;
29
(4)
:
319-359
.
-
Siljander
P R,
Platelet-derived microparticles - An updated perspective. Thromb Res.
2011;
56
(2)
:
70152-70155
.
View Article Google Scholar -
Stepien
E,
Partyka
L,
Janna
G,
Anna
Joanna G,
Jarosaw
G,
.
S,
Potential role of platelet derived microparticles and their aggregates in atherosclerotic plaque vascularization Frontiers in CardioVascular Biology , London 30th March – 1st April 2012 Second Congress of the ESC Council on Basic Cardiovascular Science PO. 2012
.
View Article Google Scholar -
Walsh
T G,
Metharom
P,
Berndt
M C,
The functional role of platelets in the regulation of angiogenesis The functional role of platelets in the regulation of angiogenesis. Platelets.
2015;
26
(3)
:
199-211
.
View Article Google Scholar -
Koenen
R R,
Aikawa
E,
Extracellular Vesicle-Mediated Processes in Cardiovascular Diseases. Front Cardiovasc Med.
2018;
5
:
133-133
.
View Article Google Scholar -
Paudel
K R,
Panth
N,
Kim
D,
Circulating Endothelial Microparticles : A Key Hallmark of Atherosclerosis Progression. Scientifica (Cairo).
2016;
2016
:
8514056
.
View Article Google Scholar -
Badimon
L,
Padr
T,
Vilahur
G,
Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Hear J Acute Cardiovasc Care.
2012;
1
(1)
:
60-74
.
View Article Google Scholar -
Mause
S F,
Hundelshausen
P,
Von
-,
Zernecke
A,
Koenen
R R,
Weber
C,
A Transcellular Delivery System for RANTES Promoting Monocyte Recruitment on Endothelium. Arterioscler Thromb Vasc Biol.
2005;
25
(7)
.
View Article Google Scholar -
Strasser
E F,
Happ
S,
Weiss
D R,
Pfeiffer
A,
Zimmermann
R,
Eckstein
R,
Microparticle detection in platelet products by three different methods. Transfusion.
2013;
53
(1)
:
156-66
.
View Article Google Scholar -
E I Jr Joseph,
Albert
T,
Robert
F,
Clinical Relevance of Microparticles from Platelets and Megakaryocytes. Curr Opin Hematol.
2010;
17
:
578-584
.
View Article Google Scholar -
Varon
D,
Shai
E,
Platelets and their microparticles as key players in pathophysiological responses. J Thromb Haemost.
2015;
13
(1)
:
40-46
.
View Article Google Scholar -
E Csongrdi,
Nagy
B,
Fulop
T,
Varga
Z,
Karnyi
Z,
Magyar
M T,
Increased levels of platelet activation markers are positively associated with carotid wall thickness and other atherosclerotic risk factors in obese patients. Thromb Haemost.
2011;
106
(4)
:
683-92
.
View Article Google Scholar -
Zhou
B,
Guo
G,
Zheng
L,
Zu
L,
Gao
W,
Microparticles as Novel Biomarkers and Therapeutic Targets in Coronary Heart Disease. Chin Med J (Engl).
2015;
20
(2)
:
267-272
.
View Article Google Scholar -
Ueba
T,
Nomura
S,
Inami
N,
Nishikawa
T,
Kajiwara
M,
Plasma Level of Platelet-Derived Microparticles Is Associated with Coronary Heart Disease Risk Score in Healthy Men. J Atheroscler Thromb.
2010;
30
(4)
:
342-351
.
View Article Google Scholar -
Koltsova
E K,
Sundd
P,
Zarpellon
A,
Ouyang
H,
Mikulski
Z,
Zampolli
A,
Genetic deletion of platelet glycoprotein Ib alpha but not its extracellular domain protects from atherosclerosis. Thromb Haemost.
2014;
112
(6)
:
1252-63
.
View Article Google Scholar -
Massberg
S,
Brand
K,
Grner
S,
Page
S,
Mller
I,
Mller
E,
A Critical Role of Platelet Adhesion in the Initiation of Atherosclerotic Lesion Formation. J Exp Med.
2002;
7
(7)
:
887-96
.
View Article Google Scholar -
Ma
Y Q,
Qin
J,
Plow
E F,
Platelet integrin alpha (IIb)beta(3): activation mechanisms. J Thromb Haemost.
2007;
5
(7)
.
View Article Google Scholar -
Li
X,
Cong
H,
Platelet-Derived Microparticles and the Potential of Glycoprotein IIb/IIIa Antagonists in Treating Acute Coronary Syndrome. Heart Inst J.
2009;
36
(2)
:
2676586-2676586
.
-
Vasina
E M,
Cauwenberghs
S,
Feijge
Mah,
Heemskerk
Jwm,
Weber
C,
Koenen
R R,
Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis.
2011;
2
:
211-211
.
View Article Google Scholar -
Larson
M K,
Tormoen
G W,
Weaver
L J,
Luepke
K J,
Patel
I A,
Hjelmen
C E,
Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am J Physiol Physiol.
2013;
304
(3)
.
View Article Google Scholar -
Venkata Krishnan
P,
Anuradha
S,
Bhattacharjee
J,
Gaiha
M,
Effects of low dose omega-3 fatty acids on platelet functions and coagulation profile in Indian patients with type 2 diabetes mellitus with vascular complications: A prospective, preliminary study. Journal, Indian Acad Clin Med.
2007;
8
(1)
:
45-52
.
-
Cohen
M G,
Rossi
J S,
Garbarino
J,
Bowling
R,
Motsinger-Reif
A A,
Schuler
C,
Insights into the inhibition of platelet activation by omega-3 polyunsaturated fatty acids: Beyond aspirin and clopidogrel. Thromb Res.
2011;
128
(4)
:
335-340
.
View Article Google Scholar -
Phang
M,
Lincz
L,
Seldon
M,
Garg
M L,
Acute supplementation with eicosapentaenoic acid reduces platelet microparticle activity in healthy subjects. J Nutr Biochem.
2012;
23
(9)
:
1128-1133
.
View Article Google Scholar -
Harris
S,
Fatty acids and serumlipoproteins : human studies. Am J Clin Nutr.
1997;
65
(5)
:
1645S-1654S
.
View Article Google Scholar -
Mozaffarian
D,
Geelen
A,
Brouwer
I A,
Geleijnse
J M,
Zock
P L,
Katan
M B,
Effect of fish oil on heart rate in humans: A meta-analysis of randomized controlled trials. Circulation.
2005;
112
(13)
:
1945-1952
.
View Article Google Scholar -
Geleijnse
J M,
Giltay
E J,
Grobbee
D E,
Donders
Art,
Kok
F J,
Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J Hypertens.
2002;
20
(8)
:
1493-1499
.
View Article Google Scholar -
Huang
T,
Zheng
J,
Chen
Y,
Yang
B,
Wahlqvist
M L,
Li
D,
High consumption of -3 polyunsaturated fatty acids decrease plasma homocysteine: A meta-analysis of randomized, placebo-controlled trials. Nutrition.
2011;
27
(9)
:
863-867
.
View Article Google Scholar -
Kiecolt-Glaser
J K,
Belury
M A,
Andridge
R,
Malarkey
W B,
Hwang
B S,
Glaser
R,
Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav Immun.
2012;
26
(6)
:
988-995
.
View Article Google Scholar -
Kander
T,
Lindblom
E,
Schott
U,
Dose-response effects of omega-3 on platelet aggregation: an observational study. J Int Med Res.
2018;
:
300060518789817-300060518789817
.
View Article Google Scholar -
Rees
D,
Miles
E A,
Banerjee
T,
Wells
S J,
Roynette
C E,
Wahle
K W,
Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans : a comparison of young. Am J Clin Nutr.
2006;
83
(2)
:
331-373
.
-
Effect of Dietary Enrichment with Eicosapentaenoic and Docosahexaenoic Acids on in Vitro Neutrophil and Monocyte Leukotriene Generation and Neutrophil Function. N Engl J Med.
1985;
86
:
1217-1224
.
View Article Google Scholar -
Aziz
N,
Azlan
A,
Ismail
A,
Alinafiah
Mohd,
Razman
S,
.
M R,
Quantitative determination of fatty acids in marine fish and shellfish from warm water of straits of malacca for nutraceutical purposes. Biomed Res Int.
2013
.
View Article Google Scholar -
Hamilton
M C,
Hites
R A,
Schwager
S J,
Foran
J A,
Knuth
B A,
Carpenter
D O,
Lipid composition and contaminants in farmed and wild salmon. Environ Sci Technol.
2005;
39
(22)
:
8622-8629
.
View Article Google Scholar -
DG
Payan,
MY
Wong,
T
Chernov-Rogan,
FH
Valone,
WC
Pickett,
VA
Blake,
WM
Gold,
EJ
Goetzl,
Alterations in human leukocyte function induced by ingestion of eicosapentaenoic acid. Journal of clinical immunology.
1986;
6
(5)
:
402-10
.
View Article Google Scholar -
S C Abreu,
M Lopes-Pacheco,
da
A L Silva,
G X Debora,
de
O Tainá Batista,
Z K Jamil,
C Lígia Lins de,
Eicosapentaenoic acid enhances the effects of mesenchymal Stromal cell therapy in experimental allergic asthma. Front Immunol.
2015;
3
(5)
:
1-12
.
View Article Google Scholar -
Chang
W L,
Azrina
A,
Sabariah
M N,
Irmi Zarina, I Loh, SP Effects of Consuming Yellowstripe Scad versus Salmon on Lipid Profile, Fasting Glucose, Body Weight Status and Blood Pressure among Healthy Overweight Malaysian Adults. Malays J Nutr.
;
3
(434-452)
.
-
Fish and omega-3: Questions and answers. https://www.heartfoundation.org.au/images/uploads/main/Programs/Consumer_QA_Fish_Omega3_Cardiovascular_Health.pdf.
.
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 8 (2019)
Page No.: 3336-3346
Published on: 2019-08-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7824 times
- View Article downloaded - 0 times
- Download PDF downloaded - 7610 times
 Biomedpress
Biomedpress





