Abstract
Introduction: Biliary atresia (BA) is the leading cause of liver fibrosis and failure in neonates with surgical jaundice, leading to poor outcome. Clinical and animal studies showing that granulocyte colony-stimulating factor (GCSF) treatment could improve liver fibrosis and cirrhosis suggest that GCSF may be offered as a low-cost intervention to improve the course of BA. This study aims to test the hypothesis that 10 µg/kg/day x 5 days of GCSF could improve liver function, reduce molecular pro-fibrotic markers and decrease liver fibrosis in a mouse model of bile duct ligation (BDL).
Methods: Balb/c mice underwent Sham surgery, or BDL for seven days followed by subcutaneous GCSF administration at 10 µg/kg/day for five consecutive days. Twelve days post-operation, blood samples were taken from the facial vein for leukocyte/neutrophil count and for measurement of serum enzymatic activities. The median lobe of the liver was acquired for total RNA and protein extraction. Moreover, the median liver lobe was used for hematoxylin-eosin staining, sirius red staining, and for visualization by immunohistochemistry (IHC).
Results: Twelve days post-operation, GCSF-treated bile duct-ligated (BDL) mice had a higher survival rate than that of placebo-treated mice (hazard ratio=1.88, p=0.084). The GCSF-treated mice had diminished liver serum transaminase activities (AST: 228.92 ± 222.67 vs. 313.46 ± 164.80 IU/L; ALP: 573.24 ± 177.89 IU/L vs. 471.75 ± 117.92 IU/L). GCSF treatment also reduced fibrosis with down-regulation of expression of pro-fibrotic markers including TGF-β1 (-2.61-fold mRNA), α-SMA (-2.46-fold mRNA; -1.88-fold protein, p<0.001) and collagen (-3.28-fold mRNA; -1.79-fold collagen deposit, p=0.0055). Moreover, GCSF treatment led to an improvement of histological grade and a reduction of extension of ductular structures caused by cholestasis (-1.77-fold CK7-positive bile ducts, p<0.0001; -2.33-fold CK7 positivity, p<0.0001).
Conclusion: Administration of GCSF (10 μg/kg/day) for five consecutive days improved the pathological condition of BDL mice. In this study, the positive effect of GCSF could be eventually surpassed due to end-stage liver disease caused from BDL in the mouse model. Further experiments are required to elucidate the effects and mechanisms of GCSF on bile obstruction.
INTRODUCTION
Biliary atresia (BA) is a rare neonatal congenital disease with a prevalence of 1/10,000 to 1/25,000 births and is characterized by obstruction to bile flow from injuries to the bile ducts. It is the most common indication for pediatric liver transplantation. Contributing factors of BA are both congenital and environmental, yet specific causes of the disease remain unknown 1,2. Untreated BA is fatal, with a median survival of 8 months of age. In BA, fibro-obliterative bile duct injuries cause intra-hepatic and extra-hepatic bile obstruction, persistent liver inflammation, fibrosis, and advanced liver failure 3,4,5. The hepatoportoenterostomy (also known as Kasai) procedure is offered to improve bile flow, but the success rate decreases with time and the majority of patients require liver transplantation for long — term survival. The transplantation option is limited in developing countries.
Granulocyte colony-stimulating factor (GCSF) has been used to reduce the risk of infection for innate or acquired neutropenia by enhancing neutrophil production with minimal adverse effects 6,7,8. GCSF was exploited as a mobilizing agent of hematopoietic stem cells (HSC s) to increase peripheral blood stem cell harvest efficiency during allogeneic or autologous stem cell transplantation 9,10 for the treatment of many diseases, such as liver disease 11,12. Moreover, compared to the labor-intensive procedure of harvesting and reinfusing stem cells, GSCF injection alone has been shown to have multiple beneficial effects on liver disease 13,14. According to systematic reviews, GCSF treatment of patients with advanced liver failure 15,16 and acute-on-chronic liver failure 17 significantly improved survival or significantly reduced short-term mortality compared to placebo. The in vivo mechanisms include the following: GCSF mobilization of HSC homing and differentiating into hepatocytes in CCl4 mice to improve liver functions 18; increase of neutrophil circulation in the peripheral blood and neutrophil maturation to decrease the risk of infection in patients with end-stage liver disease 13,19; reduction of CD8 T cells and increase of regulatory T cells (T-regs) 19; and Kupffer cell activation to reduce inflammation in the injured liver 20. Furthermore, GCSF promotes endogenous repair mechanisms, increasing the number of proliferating hepatocytes in CCl4 mice 21 and enhanc ing proliferation of liver progenitor cells (oval cells) 22. These lines of evidence suggest that GCSF could be used to support the failing liver in BA.
In this study, we performed bile duct ligation (BDL) of Balb/c mice as a model of cholestatic liver disease to test the hypothesis that GCSF improves liver function and decreases fibrosis of intrahepatic biliary diseases, such as BA.
MATERIALS AND METHODS
Mouse strain
The study was approved by our Institutional Ethics Committee (Laboratory of Stem Cell Research and Application, University of Science, Vietnam National University (VNU)-Ho Chi Minh City (HCMC)). Healthy, 8-week old male Balb/c mice from Pasteur Institute in HCMC (Vietnam) were kept in a stable environment of 12 hours light-dark cycle in the micro-ventilation cage system (THREE-SHINE Inc., Korea) with ad libitum access to food and water, and were acclimated for 1 week prior to the operation.
Bile duct ligation (BDL) and experiment design
In this study, the BDL procedure was performed as described by Carmen Tag et al. 23. The common bile duct was ligated between sutures and divided with care to avoid injury to the portal vein and the pancreas. Intramuscular (i.m.) administration of 10 mg/kg Ilium xylazil-20 (Troy Laboratories, Australia) was used as a sedative, followed by 7 mg/kg of Zoletil (Virbac, France). Lincomycin (Vemedim, Vietnam) at 20 mg/kg x 2 doses/day was given for 3 days i.m. to prevent post-surgery infections. BDL mice with visible jaundice at 7 days post-operation (d.p.o) were divided into three treatment groups (n=5):
(1) Sham-surgery treated with 200 μl/day of NaCl (0.9% solution);
(2) BDL with Placebo as 200 μl/day of NaCl (0.9% solution); and
(3) BDL + GCSF (10 µg/kg/day; Neupogen® Syringe (Filgrastim) (Roche, Switzerland)).
All treatments were administered subcutaneously (s.c.) starting on d.p.o 8 for 5 consecutive days, and mice were humanely sacrificed the day after the last dose of GCSF (on day 13 post surgery).
Blood sample preparation
Blood samples were taken from the facial vein. Leukocyte and neutrophil counts were calculated by trained and blinded observers using the blood film technique. In brief, a drop of the blood sample was smeared upon a glass slide, air-dried for 3 minutes, fixed in absolute methanol for 5 minutes, and then stained with Giemsa's azure eosin methylene blue solution (Merck Millipore, Germany). Serum aspartate aminotransferase, alkaline phosphatase, and albumin levels were assayed using the GOT (ASAT) IFCC mod. liquiUV kit (HUMAN Diagnostics, Germany), QuantiChrom™ Alkaline Phosphatase Assay Kit (BioAssay Systems, USA), and QuantiChrom™ BCG Albumin Assay Kit (BioAssay Systems, USA), respectively. Data were acquired and analyzed by the DTX 880 Multimode Detector system (Beckman Coulter, USA).
Real-time RT-PCR analysis
Total RNA was extracted from the median lobe of the liver with easy-spin (DNA-free) Total RNA Extraction Kit (iNtRON, Korea). The cDNA was synthesized using SensiFAST™ cDNA Synthesis Kit (Bioline, UK). Real-time RT-PCR analyses were performed using SensiFAST™ SYBR® Hi-ROX Kit (Bioline, UK), and then data were acquired and analyzed on the LightCycler® 480 Instrument II (Roche Life Science, USA) in reference to the Livak's 2-ΔΔCt method 24. Primers used in this study are shown below:
| Gene | Forward (5’-3’) | Reverse (5’-3’) | ID |
| TGF β1 | TGACGTCACTGGAGTTGTACGG | GGTTCATGTCATGGATGGTGC | NM_011577.2 |
| α – SMA | GCATCCACGAAACCACCTA | CACGAGTAACAAATCAAAGC | NM_007392.3 |
| Collagen type I α1 | CCTGGACGCCATCAAGGTCTAC | CCAAGTTCCGGTGTGACTCG | NM_007742.4 |
| Gapdh | TCACCATCTTCCAGGAGC | TCACCATCTTCCAGGAGC | NM_001289726.1 |
Immunohistochemistry
For immunohistochemistry and Western blot analysis, the primary antibodies used were anti-cytokeratin 7 (ab181598) and smooth muscle actin-a lpha (ab15734) (both from Abcam, MA, USA), β-actin (13E5, Cellsignaling, MA, USA). The secondary antibody was horseradish peroxidase-conjugated secondary goat-anti-rabbit (ab6721, Abcam, MA, USA). Liver tissue used in these analyses was from the median lobe.
The IHC procedure was previously described 25. In brief, slides were blocked in goat serum blocking buffer (2% Goat serum, 1% BSA, 0.1% Triton X100, 0.05% Tween-20 in phosphate-buffered saline, pH 7.2-7.4, with 0.05 % sodium azide) before incubating with primary α-smooth muscle actin (α-SMA) (1:200) or anti-cytokeratin 7 (CK7) antibody (1:5000). Slides were incubated with peroxidase blocking buffer, i.e. 3% hydrogen peroxide (H 2 O 2 ) in PBS, for 10 minutes at RT to block intracellular peroxidase, and then incubated with secondary antibody (1:500) in Tris-buffered saline (TBS) solution with 1% bovine serum albumin (BSA) for 1 h at RT. Immunocomplexes were developed using the ACE kit (Sigma-Aldrich, St Louis, MO). Hematoxylin-Gill III (Merck Millipore, Germany) was used for counterstaining. To quantify the CK7-positive ductal structures around the portal area, 10 random images/slide x 3 slides/mice were captured at x200 magnification. The CK7-positive bile duct densities were calculated as the number of CK7-positive bile ducts divided by tissue surface area.
Western Blot
Liver tissue was instantly frozen in liquid nitrogen and preserved at -80o C until use. Ten mg of tissue was digested and then protein concentration was identified by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, USA) using DTX 880 Multimode Detector system (Beckman Coulter, USA). Protein samples were subjected to SDS-PAGE on the SE 250 Minivertical Unit (GE Healthcare Life Sciences, USA) at 100 V for 3 h. T he protein bands were transferred into the Immun-Blot® PVDF Membrane (BIO-RAD, Singapore) in the Western blot blotting tank (GE Healthcare Life Sciences, USA) at 100 V for 3 h. The membrane was pre-blocked with TBS (containing 0.1% Tween 20), 5% skimmed milk for 30 minutes before incubation with the appropriate antibodies (primary α-SMA (1:1000) or CK7 (1:5000) or beta-actin (1:6000)) dissolved into TBS (containing 0.1% Tween 20) and 0.5% skimmed milk at 4 o C for 12 h. Signals were developed using Clarity™ Western ECL Kit (BIO-RAD, USA) and visualized by Azure c300 Chemiluminescent Western Blot Imaging System (LifeGene, Israel). The band intensity was measured by the free software Image Studio™ Lite (LI-COR Biosciences, USA).
Histological analysis
Mouse liver tissue was obtained from the median lobe, fixed in 4% paraformaldehyde (Merck Millipore, Germany), and sectioned from the core of the tissue mass. The tissue was paraffin-embedded and cut into 4 µm slices, with each cross-section approximately 1 mm apart.
Hematoxylin-Eosin staining : Liver slides were stained with Papanicolaou's solution 1a (also called Harris' hematoxylin solution) (Merck Millipore, Germany) and Eosin Y (0.5 %) aqueous solution (Merck Millipore, Germany) according to the protocols afore mentioned.
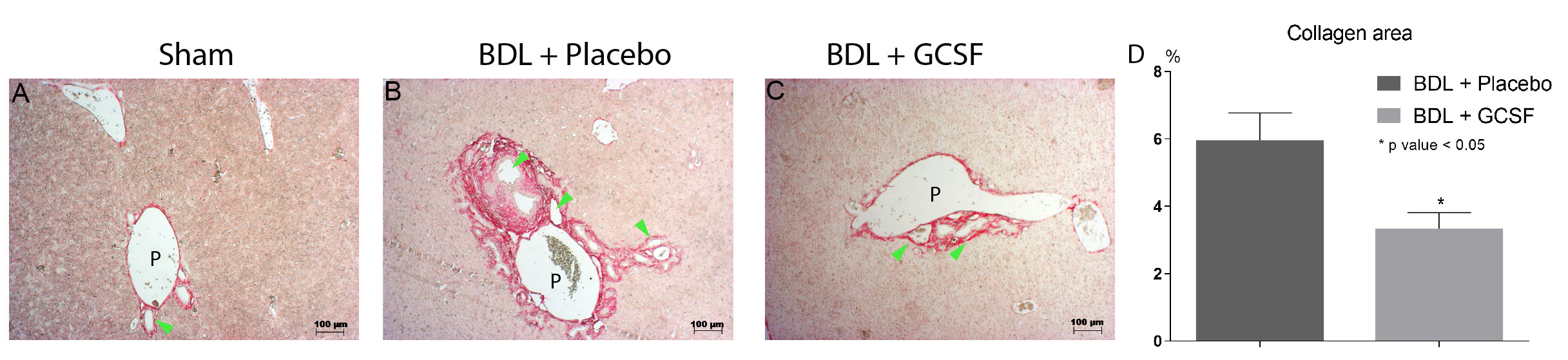
Picrosirius red staining and relative-quantification of the collagen-positive area: Liver slides were stained with Picrosirius Red Stain Kit (Polysciences, PA, USA) according to the manufacturer’s instructions. Collagen accumulation around the portal areas and inside the liver parenchyma (excluding the blood vessels) of the liver lobule (at 10 lobules/slice x 5 slices/mouse) was determined by switching the captured images into the green channel in the RGB stack mode to highlight the red-stained collagen (ImageJ 1.51r software). A trained and blinded observer analyzed the area percentage of positive sites.
Statistical analysis
Data in this study were analyzed and performed by the software GraphPad Prism 6 (GraphPad Inc ., USA). For survival analysis, the Log-rank (Mantel-Cox) test was used. Differences were considered as statistically significant if p-value ≤ 0.05. For boxplot comparison, the difference between medians (DBM) and overall visible spreading (OVS) was calculated. The two groups were considered significantly different if DBM/OVS was ≥ 50%.
The calculations were as follows: difference between medians (DBM)= median(1)-median(2); overall visible spread (OVS)=Q3-Q1 in which Q3 is the larger 3rd quartile value and Q1 is the smaller 1st quartile value.
RESULTS
Biliary obstruction diminishes liver function of BDL mice
At 7 days, BDL mice showed jaundice and ruffling (Figure 1a), and visible bile duct obstruction with full gallbladder at sacrifice (Figure 1b). The serum activity of aspartate transaminase (AST) significantly increased (p=0.0022), with a decline in albumin (ALB) concentration, when compared to those of sham mice (Figure 1c) (p=0.03).
GCSF administration increases leukocyte/neutrophil count and reduces the mortality rate of BDL mice
After 5 days of GCSF, consistent with GCSF ’s effect on mobilizing bone marrow cells, the total serum leukocyte count in GCSF-treated mice reached 32.72±13.49 x 106 cells/ml (n=5), while that of the placebo was 10.46 ± 4.99 x106 cells/ml (n=4) (p<0.05) (Figure 2a). The neutrophil count in GCSF-treated mice was 27.83±14.41x106 cells/ml (n=5), compared to 6.48±2.87 x 106 cells/ml (n=4) in the placebo group (p<0.05) (Figure 2b). The corresponding median survival of GCSF-treated BDL mice (n=10 for each group) was 13.0 days, as compared to 10.5 days for placebo-BDL mice, with a hazard ratio (log -rank) of 1.88 (Figure 2c) (p= 0.08 nearing statistical significance).
GCSF reduces elevation of serum transaminases in BDL mice
There was a decrease in serum aspartate aminotransferase (AST) concentration in BDL mice treated with GCSF (median serum AST: 116.33 IU/L), compared to those BDL mice treated with placebo (median serum AST: 297.76 IU/L) (DBM/OVS=51.23%) (Figure 3a). This was not seen with serum ALP or albumin values (Figure 3b, c). Though positive results were seen, these indicators did not fully reflect the disease condition in treated mice and were not significant different (Figure 3, p>0.05); this was expected as bile duct obstruction was fixed26.
GCSF administration reduces the expression of pro-fibrotic markers in BDL mice
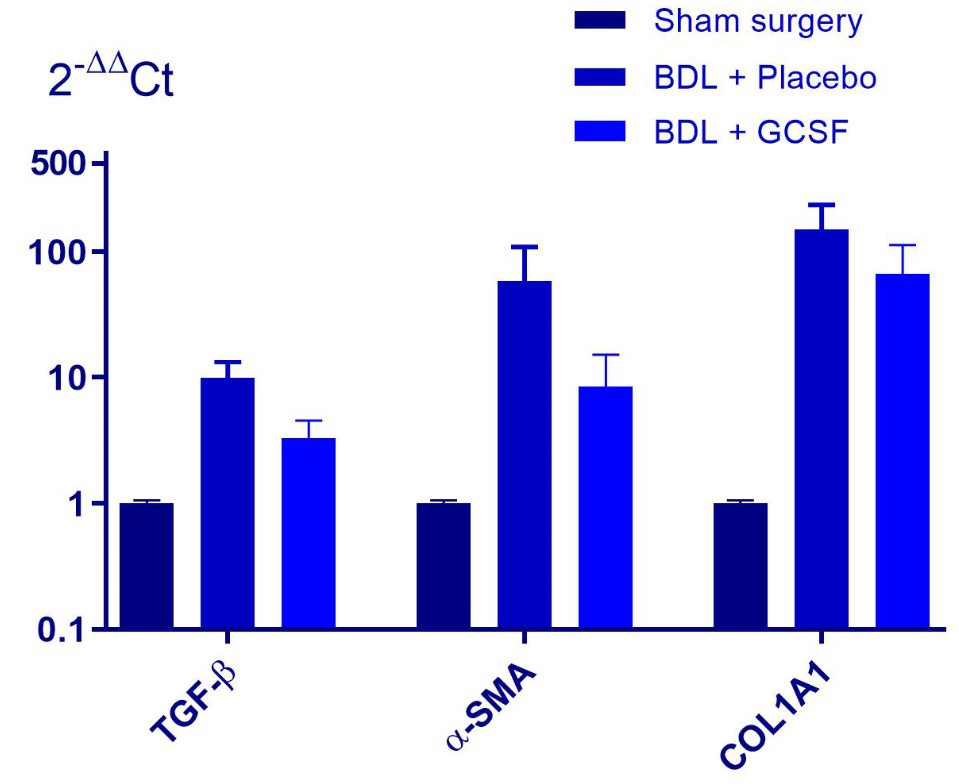
Down-regulation of mRNA levels of pro-fibrotic genes in GCSF-treated BDL mice were observed, with notable decrease in the levels of TGF-β mRNA (2.61 fold-change, DBM/OVS=85.99%), α-SMA (2.46 fold-change, DBM/OVS=22.83%), and col1a1 (3.28 fold-change, DBM/OVS=69.41%) (Figure 4). A parallel reduction in the amount of collagen deposit by 1.79-fold (p=0.006) was seen around and across portal areas (bridging fibrosis) of the GCSF treated BDL mice compared to placebo (Figure 5).
Similarly, consistent with the down regulation of α-SMA expression, Western blot data showed a 10.46±0.36 and 5.56±0.81 fold-change for BDL and GCSF-BDL groups, respectively, compared to that for sham mice (p<0.0001) (Figure 6). Immunostaining confirmed a decreased periportal α-SMA expression in GCSF-treated liver of BDL mice (Figure 7).
GCSF administration improves histology injury and reduces ductal reaction
There was significant improvement in the liver histology of GCSF-treated BDL mice compared to that of BDL mice, with fewer infarction zones (Figure 8) from BDL bile necrosis. Moreover, as previously mentioned, there was less bridging fibrosis (Figure 5d: -1.79-fold change, p=0.006) around the expansion areas of ductal structures (Figure 9d: -1.77-fold change, p<0.0001) in the GCSF-treated BDL mice.
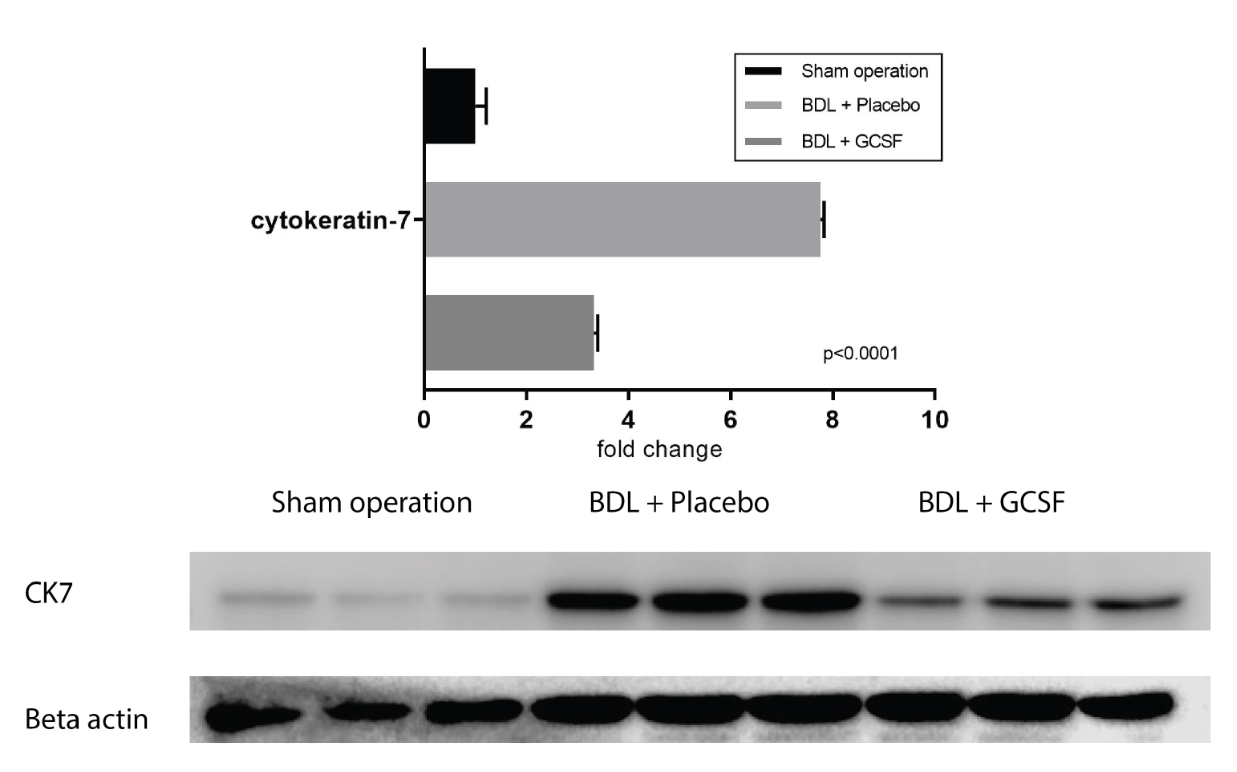
In compensation to hepatic injury, bile duct progenitor cells proliferate to expand the ductal structures 27. This was seen in the portal areas of BDL liver with a high number of cytokeratin-7 (CK-7) positive newly formed ductal structures (Figure 9b) (41.12 ± 1.1 bile ducts/mm2). GCSF treatment reduced the number of CK7 positive bile ducts (23.2 ± 2.75 bile ducts/mm2) (1.77-fold change, p<0.0001), as well as decreased whole liver CK7 protein levels (2.33-fold reduction, p<0.0001) (Figure 10).
DISCUSSION
In this study, bile duct ligation (BDL) leads to obstructive jaundice, hepatomegaly, increased hepatic transaminase levels, and decreased liver functions (such as albumin levels). Since Balb/c mice are more vulnerable to hepatic fibrosis 28 and liver injury, with a high mortality by 14 days of surgery compared to other strains of mice, this study was limited to a 12-day course after mice exhibited liver injury by the 7th day and after completion of the 5 doses of GCSF treatment. In this study, administration of 5 doses of 10 µg/kg/day to BDL Balb/c mice increased the leukocyte and neutrophil counts in peripheral blood of these mice, compared to that for placebo-treated mice (p<0.05). Our results showed that administration of GCSF improved the mortality in GCSF-treated BDL mice compared to the placebo (hazard ratio=1.88)27. In this study, GCSF-treated BDL mice had low AST activities compared to placebo-treated BDL mice, suggesting a less injured liver 21,27. Furthermore, GCSF diminished the liver fibrosis via down regulation of pro-fibrotic genes, such as TGF-β1, α-SMA, and col1a1. This was consistent with reduction of α-SMA protein level (confirmed by IHC and Western blot) and collagen deposit (confirmed by Sirius red staining) 21.
This model of liver injury adds to the list of other experimental models of liver injury, including partial hepatectomy in rats 22, radiation 29, acute failure from thioacetamide (TAA) 30, D-galactosamine31, CCl4 32 and NAFLD 33 publications show the beneficial effects of GCSF on the attenuation of hepatic injury (adjust references). Underlying the attenuated hepatic injury are various phenomena, including the reduction of the inflammatory response, the promotion of endogenous repair 21, increase in hepatocyte proliferation 34,35 with increased PI3K/Akt pathways 33, development of oval cell proliferation 22, and reduction in the apoptotic drive33.
During liver injury, a correlation between ductular reaction and fibrogenesis, presumably from the extracellular synthesis of the newly forming bile ducts, have been reported 36,37,38. Inhibition of the ductular response reduced liver fibrosis in MDR2 -/- mice 39. In this study, G-CSF significantly reduced cytokeratin-7 (CK7) expression and the number of CK7-positive ductal structures in BDL mice, providing a novel effect of GCSF on improving liver injury by diminishing the ductular reaction. Future studies into the mechanism of GCSF protective effects in liver injury should target the relationship between GCSF and the ductular response. It is worth noting that the histopathological features of biliary atresia include portal fibrosis and inflammation, but also ductular proliferation 39. Therefore, these evidence provides a rationale for the use of GCSF as an intervention for BA.
CONCLUSION
In this experimental model of intermediate-term liver damage by obstructive BDL injury, post-injury treatment with GCSF improved the hepatic outcome of bile duct-ligated mice, notably a reduction in hepatic fibrosis and its association with down regulation of the ductular reaction. GCSF may be useful as a treatment for liver fibrosis in BA disease.
ABBREVIATIONS
ALB: Albumin
ALP: Alkaline photphatase
AST: Aspartate transaminase
BA: Biliary Atresia
BDL: Bile duct ligation
CK7: Cytokeratin-7
GCSF: Granulocyte colony stimulating factor
HSC: Hematopoietic stem cell
IHC: Immunohistochemistry
MELD: Model for End-stage Liver Disease
NAFOSTED: National Foundation for Science and Technology Development
αSMA: alpha Smooth Muscle Actin
TGFβ: Transforming Growth Factor beta
AUTHOR CONTRIBUTION
Huy Quang Do conducted the experiments and composed the manuscript. Trinh Van Le, Minh Thanh Dang, Tien-Trieu Pham-Le and Luan Van Chan carried out the experiments and analyzed the liver function, gene-expression and histology. Khon Chan Huynh performed and analyzed the protein expression. Ai-Xuan Le Holterman is senior researcher, advices on the design of experimental models and on data analyses and revise the manuscript. Nhung Hai Truong made substantial contributions to the ideas, experiment design , interpretation of data, and submission.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENT
This research is funded by Vietnam National Foundation for Science & Technology Development (NAFOSTED) under grant number 108.05-2017.30 and Science & Technology Incubator Youth Program, managed by the Center for Science and Technology Development, Ho Chi Minh Communist Youth Union.
References
-
Lakshminarayanan
B.,
Davenport
M.,
Biliary atresia: A comprehensive review. J Autoimmun.
2016;
73
:
1-9
.
View Article PubMed Google Scholar -
Nakamura
K.,
Tanoue
A.,
Etiology of biliary atresia as a developmental anomaly: recent advances. J Hepatobiliary Pancreat Sci.
2013;
20
(5)
:
459-64
.
View Article PubMed Google Scholar -
Bates
M.D.,
Bucuvalas
J.C.,
Alonso
M.H.,
Ryckman
F.C.,
Biliary atresia: pathogenesis and treatment. Semin Liver Dis.
1998;
18
(3)
:
281-93
.
View Article PubMed Google Scholar -
Hays
D.M.,
Snyder
W.H.,
Life-span in untreated biliary atresia. Surgery.
1963;
54
(2)
:
373-5
.
PubMed Google Scholar -
Adelman
S.,
Prognosis of uncorrected biliary atresia: an update. J Pediatr Surg.
1978;
13
(4)
:
389-91
.
View Article PubMed Google Scholar -
Panopoulos
A.D.,
Watowich
S.S.,
Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and `emergency' hematopoiesis. Cytokine.
2008;
42
(3)
:
277-88
.
View Article PubMed Google Scholar -
Carr
R.,
Modi
N.,
Dore
C.,
G-CSF and GM-CSF for treating or preventing neonatal infections. The Cochrane database of systematic reviews.
2003;
2003
(3)
:
Cd003066
.
-
Kraj
L.,
Krawczyk-Lipiec
J.,
Górniewska
J.,
Orlik
G.,
Efficacy and safety of biosimilar filgrastim in primary and secondary prevention of febrile neutropenia. Biomed Rep.
2017;
7
(2)
:
143-7
.
View Article PubMed Google Scholar -
Bendall
L.J.,
Bradstock
K.F.,
G-CSF: from granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev.
2014;
25
(4)
:
355-67
.
View Article PubMed Google Scholar -
Publicover
A.,
Richardson
D.S.,
Davies
A.,
Hill
K.S.,
Hurlock
C.,
Hutchins
D.,
Use of a biosimilar granulocyte colony-stimulating factor for peripheral blood stem cell mobilization: an analysis of mobilization and engraftment. Br J Haematol.
2013;
162
(1)
:
107-11
.
View Article PubMed Google Scholar -
Salama
H.,
Zekri
A.R.,
Medhat
E.,
Al Alim
S.A.,
Ahmed
O.S.,
Bahnassy
A.A.,
Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther.
2014;
5
(3)
:
70
.
View Article PubMed Google Scholar -
Terai
S.,
Ishikawa
T.,
Omori
K.,
Aoyama
K.,
Marumoto
Y.,
Urata
Y.,
Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells.
2006;
24
(10)
:
2292-8
.
View Article PubMed Google Scholar -
Duan
X.Z.,
Liu
F.F.,
Tong
J.J.,
Yang
H.Z.,
Chen
J.,
Liu
X.Y.,
Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol.
2013;
19
(7)
:
1104-10
.
View Article PubMed Google Scholar -
Duan
X.Z.,
Liu
F.F.,
Tong
J.J.,
Yang
H.Z.,
Chen
J.,
Liu
X.Y.,
Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol.
2013;
19
(7)
:
1104-10
.
View Article PubMed Google Scholar -
Yang
Q.,
Yang
Y.,
Shi
Y.,
Lv
F.,
He
J.,
Chen
Z.,
Effects of Granulocyte Colony-Stimulating Factor on Patients with Liver Failure: a Meta-Analysis. J Clin Transl Hepatol.
2016;
4
(2)
:
90-6
.
PubMed Google Scholar -
Kumar
A.,
Sharma
P.,
Arora
A.,
Granulocyte colony-stimulating factor for advanced liver disease: a meta-analysis. J Hepatol.
2017;
66
(1)
:
132
.
View Article Google Scholar -
Chavez-Tapia
N.C.,
Mendiola-Pastrana
I.,
Ornelas-Arroyo
V.J.,
Noreña-Herrera
C.,
Vidaña-Perez
D.,
Delgado-Sanchez
G.,
Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol.
2015;
14
(5)
:
631-41
.
View Article PubMed Google Scholar -
Tsolaki
E.,
Athanasiou
E.,
Gounari
E.,
Zogas
N.,
Siotou
E.,
Yiangou
M.,
Hematopoietic stem cells and liver regeneration: differentially acting hematopoietic stem cell mobilization agents reverse induced chronic liver injury. Blood Cells Mol Dis.
2014;
53
(3)
:
124-32
.
View Article PubMed Google Scholar -
Garg
V.,
Garg
H.,
Khan
A.,
Trehanpati
N.,
Kumar
A.,
Sharma
B.C.,
Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology.
2012;
142
(3)
.
View Article PubMed Google Scholar -
Busch
C.J.,
Wanner
G.A.,
Menger
M.D.,
Vollmar
B.,
Granulocyte colony-stimulating factor (G-CSF) reduces not only gram-negative but also gram-positive infection-associated proinflammatory cytokine release by interaction between Kupffer cells and leukocytes. Inflammation research : official journal of the European Histamine Research Society.
2004;
53
(5)
:
205-10
.
-
Yannaki
E.,
Athanasiou
E.,
Xagorari
A.,
Constantinou
V.,
Batsis
I.,
Kaloyannidis
P.,
G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol.
2005;
33
(1)
:
108-19
.
View Article PubMed Google Scholar -
Piscaglia
A.C.,
Shupe
T.D.,
Oh
S.H.,
Gasbarrini
A.,
Petersen
B.E.,
Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology.
2007;
133
(2)
:
619-31
.
View Article PubMed Google Scholar -
Tag
C.G.,
Sauer-Lehnen
S.,
Weiskirchen
S.,
Borkham-Kamphorst
E.,
Tolba
H.,
Bile Duct Ligation in Mice: Induction of Inflammatory Liver Injury and Fibrosis by Obstructive Cholestasis. JoVE (Journal of Visualized Experiments).
2015;
96
:
e52438
.
-
Livak
K.J.,
Schmittgen
T.D.,
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods.
2001;
25
(4)
:
402-8
.
View Article PubMed Google Scholar -
Truong
N.H.,
Nguyen
N.H.,
Le
T.V.,
Vu
N.B.,
Huynh
N.,
Nguyen
T.V.,
Comparison of the Treatment Efficiency of Bone Marrow-Derived Mesenchymal Stem Cell Transplantation via Tail and Portal Veins in CCl4-Induced Mouse Liver Fibrosis. Stem Cells Int.
2016;
2016
:
5720413
.
View Article PubMed Google Scholar -
Abshagen
K.,
König
M.,
Hoppe
A.,
Müller
I.,
Ebert
M.,
Weng
H.,
Pathobiochemical signatures of cholestatic liver disease in bile duct ligated mice. BMC Syst Biol.
2015;
9
(1)
:
83
.
View Article PubMed Google Scholar -
Strazzabosco
M.,
Fabris
L.,
Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol.
2012;
56
(5)
:
1159-70
.
View Article PubMed Google Scholar -
Walkin
L.,
Herrick
S.E.,
Summers
A.,
Brenchley
P.E.,
Hoff
C.M.,
Korstanje
R.,
The role of mouse strain differences in the susceptibility to fibrosis: a systematic review. Fibrogenesis Tissue Repair.
2013;
6
(1)
:
18
.
View Article PubMed Google Scholar -
Zhang
L.,
Kang
W.,
Lei
Y.,
Han
Q.,
Zhang
G.,
Lv
Y.,
Granulocyte colony-stimulating factor treatment ameliorates liver injury and improves survival in rats with D-galactosamine-induced acute liver failure. Toxicol Lett.
2011;
204
(1)
:
92-9
.
View Article PubMed Google Scholar -
Li
N.,
Zhang
L.,
Li
H.,
Fang
B.,
Human CD34+ cells mobilized by granulocyte colony-stimulating factor ameliorate radiation-induced liver damage in mice. Stem Cell Res Ther.
2010;
1
(3)
:
22
.
View Article PubMed Google Scholar -
Esmaili
M.,
Qujeq
D.,
Yoonesi
A.A.,
Feizi
F.,
Ranaee
M.,
Effects of associated SCF and G-CSF on liver injury two weeks after liver damage: A model induced by thioacetamide administration. Mol Biol Res Commun.
2014;
3
(2)
:
141-7
.
PubMed Google Scholar -
Zhang
L.,
Kang
W.,
Lei
Y.,
Han
Q.,
Zhang
G.,
Lv
Y.,
Granulocyte colony-stimulating factor treatment ameliorates liver injury and improves survival in rats with D-galactosamine-induced acute liver failure. Toxicol Lett.
2011;
204
(1)
:
92-9
.
View Article PubMed Google Scholar -
Qujeq
D.,
Abassi
R.,
Faeizi
F.,
Parsian
H.,
Faraji
A.S.,
Taheri
H.,
Effect of granulocyte colony-stimulating factor administration on tissue regeneration due to carbon tetrachloride-induced liver damage in experimental model. Toxicol Ind Health.
2013;
29
(6)
:
498-503
.
View Article PubMed Google Scholar -
Nam
H.H.,
Jun
D.W.,
Jang
K.,
Saeed
W.K.,
Lee
J.S.,
Kang
H.T.,
Granulocyte colony stimulating factor treatment in non-alcoholic fatty liver disease: beyond marrow cell mobilization. Oncotarget.
2017;
8
(58)
:
97965-76
.
View Article PubMed Google Scholar -
Kimura
M.,
Yamada
T.,
Iwata
H.,
Sekino
T.,
Shirahashi
K.,
Yoshida
N.,
Preoperative granulocyte-colony stimulating factor (G-CSF) treatment improves congested liver regeneration. J Surg Res.
2010;
158
(1)
:
132-7
.
View Article PubMed Google Scholar -
Rókusz
A.,
Veres
D.,
Szücs
A.,
Bugyik
E.,
Mózes
M.,
Paku
S.,
Links Between Hepatic Fibrosis, Ductular Reaction, and Progenitor Cell Expansion. Gastroenterology.
2017;
146
(2)
:
349-56
.
View Article Google Scholar -
Williams
M.J.,
Clouston
A.D.,
Forbes
S.J.,
Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology.
2014;
146
(2)
:
349-56
.
View Article PubMed Google Scholar -
Sato
K.,
Marzioni
M.,
Meng
F.,
Francis
H.,
Glaser
S.,
Alpini
G.,
Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology.
2019;
69
(1)
:
420-30
.
View Article PubMed Google Scholar -
McDaniel
K.,
Wu
N.,
Zhou
T.,
Huang
L.,
Sato
K.,
Venter
J.,
Key Histopathologic Features of Liver Biopsies That Distinguish Biliary Atresia From Other Causes of Infantile Cholestasis and Their Correlation With Outcome: A Multicenter Study. The American journal of surgical pathology.
2016;
40
(12)
:
1601-15
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 6 (2019)
Page No.: 3222-3232
Published on: 2019-06-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 9558 times
- Download PDF downloaded - 1475 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress