Abstract
Background: Recent evidence has suggested that oxidative stress may play a role in the pathophysiology of migraine. In this study, we aimed to evaluate the oxidative-antioxidative status in sera of migraine patients from an Iranian population during migraine attacks.
Methods: This study recruited 46 migraine patients with or without aura and 45 sex- and age-matched healthy controls. The levels of protein carbonyl (PC), malondialdehyde (MDA) and total oxidants status (TOS) were measured as the indicators of oxidative stress. The levels of total thiols (T-SH), reduced glutathione (GSH) and total antioxidant capacity (TAC) were determined as markers of antioxidant status. Oxidative stress index (OSI) was calculated by dividing TOS to TAC.
Results: The serum levels of MDA (4.10 +/- 0.70 vs. 3.20 +/- 0.62, P = 0.003), TOS (18.46 +/- 4.06 vs. 16.21 +/- 3.67, P = 0.007) and OSI (1.54 +/- 0.60 vs. 1.22 +/- 0.46, P = 0.006) were significantly higher in migraine group compared to controls, however, no statistically significant differences of PC levels were found between migraine patients and controls (0.615 +/- 0.161 vs. 0.517 +/- 0.126, P = 0.1). In contrast, the levels of T-SH (273 +/- 51.71 vs. 310.88 +/- 53.32, P = 0.001), GSH (28.08 +/- 6.20 vs. 32.13 +/- 5.8, P = 0.002) and TAC (1.27 +/- 0.226 vs. 1.41 +/- 0.26, P = 0.01) were significantly lower in migraine patients compared to healthy controls.
Conclusion: Our study showed higher levels of oxidative stress and lower levels of antioxidant status in migraine group compared to controls, which indicates the possible role of oxidative stress in triggering migraine attacks.
Introduction
Migraine is a prevalent neurological disorder characterized by severe episodic headaches with systemic or neurological symptoms1,2. There are two major types of migraines: migraines with aura and migraines without aura. Aura is experienced by about a third of migraine patients and consists of a series of transient neurological symptoms that occur in the visual system or other senses before the onset of migraine headache2. Although several genetic and environmental factors have been shown to be associated with the presence or development of migraine3, molecular mechanisms of migraine attacks have not been clearly identified. One of the major factors lowering the threshold of the first headache symptom is the impairment of mitochondrial oxidative metabolism4. In this regard, it has been proposed that nitric oxide and oxidative stress may play a central role in migraine headache by deregulating brain blood flow5. In fact, oxidative stress originates from the imbalance between the production of reactive oxygen species (ROS) and antioxidants6. ROS are reactive oxidant molecules containing oxygen, which are formed at relatively low levels in all cells during normal metabolism and their physiological levels are necessary to maintain normal cell function7. The concentration of all oxidants in biological samples is referred to as total oxidant status (TOS)8.
The additional amounts of ROS are removed by free radical-scavenging enzymes such as superoxide dismutase, catalase, and glutathione peroxidase 7. Also, there are non-enzymatic antioxidants such as ascorbic acid, vitamin E, uric acid, bilirubin, reduced glutathione (GSH), and various thiol groups on protein molecules that can act to neutralize ROS9. The reduced sulfhydryl groups of proteins and non-protein compounds are collectively known as total thiols10 and all antioxidants present in the serum are referred to as total antioxidant capacity (TAC)11. When the levels of ROS increase beyond the antioxidant capacity of the cell, a condition known as oxidative stress occurs. In such situation, free radicals may damage cellular proteins, lipids, and nucleic acids, which can potentially trigger the development of various human diseases including migraine12,13. Oxidative damage to proteins can generate protein carbonyls (PC)14 and decrease free sulfhydryl groups of proteins and other thiol-containing compounds (or total thiols). The oxidation of lipids produces malondialdehyde (MDA), which is measured as one of the indicators of oxidative stress15. In some diseases, the main pathophysiologic basis may come from the activation of oxidative stress and inactivation of antioxidant processes16,17. Studies have shown that high levels of oxidative stress are associated with an increase in migraine risk. Given the fact that both genetics and environmental factors can affect migraine and oxidative stress18, this study was designed to examine the association of non-enzymatic oxidative stress markers with migraine headache in an Iranian population sample.
Methods
Patients
This case-control study was approved by the Ethics Committee of Zahedan University of Medical Sciences and recruited 46 migraine patients with or without aura during attacks presenting at public or private clinics in Zahedan, Iran in 2014. Migraine was diagnosed based on the International Headache Society (IHS) criteria, 2nd edition1. This study excluded patients with the presence of other types of headaches, history of diabetes mellitus, tuberculosis, malaria, history of any types of malignancies, depression, underlying active systemic or inflammatory disorders, or history of using any vitamins or antioxidant supplements. Meanwhile, 45 sex- and age-matched healthy individuals without any history of migraine from medical students and university personnel were selected as the controls. An informed written consent form was sent to all participants. Following overnight fasting, blood samples were taken from cases and controls. Coagulation serum samples were obtained by centrifugation, which were then aliquoted for each test and kept at -70 °C until analysis. Each analysis was conducted in duplicates under similar conditions on the same day.
Biochemical assays
Chemical materials
Potassium persulfate, xylenol orange, sulfuric acid, sodium chloride, hydrogen peroxide, glycerol, sulfosalicylic acid (SSA), sodium phosphate, orthophosphoric acid, ferric chloride and TBA (2-thiobarbituric acid: 4, 6- dihydroxy-2-mercaptopyrimidine) were purchased from Merck. ABTS [2, 2-azinobis (3-ethylbenzothiazoline-6-sulfonate)], Trolox, Ortho-dianisidine, Malondialdehyde standard (1,1,3,3-tetramethoxypropane), DTNB (5,5'-dithiobis-2-nitrobenzoic acid) and reduced glutathione (GSH) were obtained from Sigma.
Total antioxidant capacity (TAC) assay
The TAC of serum was measured based on the discoloration of 2, 2-azinobis 3-ethylbenzothiazoline-6-sulfonate radical cation (ABTS*+) by antioxidants present in the sample19. ABTS*+ was produced by incubation of 7 mM ABTS aqueous solution with 2.5 mM potassium persulfate and allowing the mixture to remain in the dark at room temperature for 12-16 hours before use. To measure sample TAC and ABTS*+, stock solution was diluted in deionized water until its absorbance at 734 nm reached 0.70. One mL of diluted ABTS*+ was added to 10 μL of serum, then the absorbance was read ten minutes after the initial mixing. The TAC was calculated using Trolox as a standard antioxidant and the result was expressed as μM Trolox.
Total oxidant status (TOS) assay
The TOS levels of the serum were determined using Erel's method that was based on the oxidation of ferrous ion-O-dianisidine complex to ferric ions by oxidants present in the sample20. The ferric ions in acidic medium formed a colored complex with xylenol orange. Briefly, a solution of 900 μL of H2SO4 25 mM containing 150 μM of xylenol orange, 140 mM NaCl and 1.35 M glycerol was mixed with 100 μl of serum or standard TOS. Then, 45 μl of solution containing 5 mM ferrous ion and 10 mM O-dianisidine in H2SO4 25 mM was added to the mixture. After 5 minutes, color intensity was measured spectrophotometrically at 560 nm. TOS were calculated from a standard curve using hydrogen peroxide as a standard TOS and the results were expressed in µM H2O2 Equivalent/L.
Oxidative stress index
Oxidative stress index (OSI) was calculated using the following equation: OSI = [TOS (µmol H2O2 Equivalent/L)/TAC (µmol Torolox Equivalent/L)] x 100.
Total thiol assay
The levels of total thiols were measured spectrophotometrically according to Hu,s method with modifications performed in our laboratory as described21. The levels of thiols in each sample was calculated by using reduced glutathione as a standard thiol and the result was expressed in μM.
Glutathione assay
At first, the serum was deproteinized with an equal volume of 5% sulfosalicylic acid (SSA) solution, centrifuged at 38000 rpm for 10 minutes at 4 ⁰C to remove precipitated proteins before being assayed for glutathione. Serum GSH was measured according to the modified method of Moron et al.22. Briefly, 200 µL of serum supernatant was mixed with 2 ml of 200 mM phosphate buffer (pH 8) containing 0.6 mM 5,5'-dithiobis (2-nitrobenzoic acid). Blank samples contained 2.5% SSA, instead of the supernatant. After 5 minutes incubation at room temperature, the absorbance was read at 405 nm against the blank sample. Reduced glutathione was dissolved in 2.5% SSA and was used as a standard. Serum GSH concentration was calculated using a standard curve and the result was expressed as μM.
Protein carbonyl assay
Before PC determination, serum protein was diluted with phosphate buffer saline (PBS) to 4 mg/mL. The levels of PC were measured by enzyme-linked immunosorbent assays (ELISA) using the commercially available kit (Cell Biolab, Inc, Company) as described by Buss et al. The results were presented as absorbance units in the ELISA reader.
MDA assay
MDA levels were measured according to the method of Uchiyama and Mihara23. Briefly, 3 mL of 1% orthophosphoric acid and 1 mL of 0.6% w/v aqueous solution of thiobarbituric acid were added to 0.5 ml of serum. The mixture was heated for 45 minutes in a boiling water bath. After cooling, the mixture was centrifuged at 3000×g for 10 minutes and the absorbance of the upper solution was determined at 535 nm against a blank sample containing 3 mL of phosphoric acid, 1 ml thiobarbituric acid and 0.5 mL PBS. The concentration of MDA was calculated using 1,1,3,3-tetramethoxypropane standard curve and was expressed in μM.
Statistics analysis
The results were presented as mean ± standard deviation (SD) for quantitative variables. For the statistical analysis, the statistical software SPSS version 21.0 (SPSS Inc., Chicago, IL) was used. Numerical variables of groups were compared by the Student’s unpaired t-test. Mann-Whitney U test was also used to compare the numerical variables of subgroups. Categorical variables were compared using the chi-squared test. P values of less than 0.05 were considered statistically significant.
Results
Demographic and clinical properties in migraine patients and healthy controls are presented in Table 1. There was no significant difference in the mean ages of migraine and control groups (27.22 ± 7.40 vs. 26.65 ± 8.20, P = 0.77). Regarding to sex distribution, 67.4% of the migraine group and 66.6% of the healthy group were female and no difference in sex distribution was observed between subjects suffering from migraine and healthy individuals (P = 0.57).
| Parameter | Healthy control (N = 45) | Migraine patients (N = 46) | P value |
| Sex (Female/Male) | 30/15 | 30/16 | 0.88 |
| Age (year) | 26.65 ± 8.20 | 27.22 ± 7.40 | 0.77 |
| Duration of migraine (years) | - | 4.56 ± 2.62 | - |
| Monthly frequency of headache | - | 5.13 ± 2.25 | - |
| Headache duration (hours) | - | 29.4±15.5 | - |
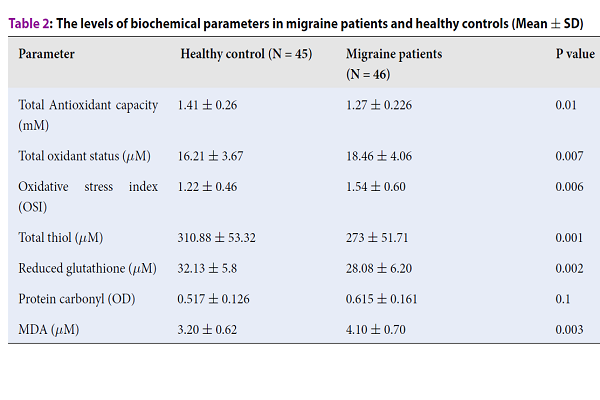
The levels of biochemical parameters in migraine patients and healthy controls are shown in Table 2. The mean serum levels of MDA (4.10 ± 0.70 vs. 3.20 ± 0.62, P = 0.003), TOS (18.46 ± 4.06 vs. 16.21 ± 3.67, P = 0.007) and OSI (1.54 ± 0.60 vs. 1.22 ± 0.46, P = 0.006) were significantly higher in migraine group than those in the healthy group. PC concentration was slightly higher in the migraine group compared to healthy controls (0.615 ± 0.161 vs. 0.517 ± 0.126), but the difference was not statistically significant (P=0.1). The levels of T--SH (273±51.71 vs. 310.88 ± 53.32, P = 0.001), GSH (28.08 ± 6.20 vs. 32.13 ± 5.8, P = 0.002) and TAC (1.27 ± 0.226 vs. 1.41 ± 0.26, P = 0.01) were significantly lower in patients with the migraine compared to healthy control individuals.
| Parameter | Healthy control (N = 45) | Migraine patients (N = 46) | P value |
| Total Antioxidant capacity (mM) | 1.41 ± 0.26 | 1.27 ± 0.226 | 0.01 |
| Total oxidant status (μM) | 16.21 ± 3.67 | 18.46 ± 4.06 | 0.007 |
| Oxidative stress index (OSI) | 1.22 ± 0.46 | 1.54 ± 0.60 | 0.006 |
| Total thiol (μM) | 310.88 ± 53.32 | 273 ± 51.71 | 0.001 |
| Reduced glutathione (μM) | 32.13 ± 5.8 | 28.08 ± 6.20 | 0.002 |
| Protein carbonyl (OD) | 0.517 ± 0.126 | 0.615 ± 0.161 | 0.1 |
| MDA (μM) | 3.20 ± 0.62 | 4.10 ± 0.70 | 0.003 |
The results of oxidative-antioxidative balance between migraine patients with and without aura are shown in Table 3. There was no statistically significant difference in serum levels of MDA (4.00 ± 0.65 vs. 4.2 ± 0.78, P = 0.056), TOS (18.28 ± 3.55 vs. 18.56 ± 4.36, P = 0.7) and OSI (1.46 ± 0.5 vs. 1.58 ± 0.64, P = 0.56) between migraine patients with and without aura. We also found no significant difference in the levels of T-SH (280.18 ± 53.07 vs. 269.56 ± 51.50, P = 0.55), GSH (29.56 ± 5.57 vs. 27.30 ± 6.46, P = 0.23), TAC (1.33 ± 0.255 vs. 1.24 ± 0.27, P = 0.3) and PC concentration (0.574 ± 0.16 vs. 0.663 ± 0.16, P = 0.3) between migraine patients with and without aura.
| Parameter | Migraine with aura (N = 15) | Migraine without aura (N = 31) | P value |
| Sex (Female/Male) | 12/4 | 18/12 | 0.35 |
| Age (year) | 28.06 ± 7.36 | 26.33 ± 7.60 | 0.52 |
| Total Antioxidant capacity (mM) | 1.33 ± 0.255 | 1.24 ± 0.27 | 0.3 |
| Total oxidant status (μM) | 18.28 ± 3.55 | 18.56 ± 4.36 | 0.7 |
| Oxidative stress index (OSI) | 1.46 ± 0.5 | 1.58 ± 0.64 | 0.56 |
| Total thiol (μM) | 280.18 ± 53.07 | 269.56 ± 51.50 | 0.55 |
| Reduced glutathione (μM) | 29.56 ± 5.57 | 27.30 ± 6.46 | 0.23 |
| Protein carbonyl (OD) | 0.574 ± 0.16 | 0.663 ± 0.16 | 0.3 |
| MDA (μM) | 4.00 ± 0.65 | 4.2 ± 0.78 | 0.56 |
Discussion
In the current study, it was hypothesized that oxidant increment and/or antioxidant depletion might be two major fundaments of the pathophysiology of migraine headache. Accordingly, we determined PC, MDA, TOS and OSI as the indicators for the activation of oxidative stress and TAC, T-SH and GSH as the markers of antioxidant status. To the best of our knowledge, this is the first to study the association between oxidative stress and migraine attack in an Iranian population sample. Overall, our results revealed that migraine attacks are associated with an increase in oxidant levels and a depletion of antioxidant defenses, which suggests a shift in oxidative-antioxidative balance towards oxidative status. The results confirmed our hypothesis and are consistent with other studies. Several studies have shown the elevated levels of MDA, a product of oxidative damage of lipids, in the blood of migraine patients when compared to controls24,25,26. In a study by Alp et al.27, the levels of total antioxidants were significantly lower in migraine patients while TOS and OSI were higher when compared to healthy individuals. Gupta et al. showed an increase in plasma levels of MDA and total antioxidant capacity in migraine patients28. Our TAC results are consistent with those of Alp et al., but did not confirm the results of Guptal et al. Moreover, the disturbance of antioxidant enzyme activities in serum and erythrocytes of migraine patients have been reported in several studies15,25.
Thiols are physiological free radical scavengers that represents approximately 53% of total antioxidants present in serum19. In the present study, low levels of GSH and T-SH in migraine patients indicate the occurrence of oxidative stress during migraine attack. Alp et al. also found lower levels of total thiols in migraine patients and detected a negative correlation between the duration of pain and thiol levels27. Similar to the study conducted by Bernecker et al., we found higher levels of PC in migraine patients compared to controls, however, the difference between the two groups was not statistically significant29. Carbonyl groups of proteins can be induced by almost all types of ROS, and high levels of these proteins are reflective of high levels of oxidative stress14.
Findings of the current and previous studies suggest that oxidative stress are likely to occur during migraine attacks. Also, there is evidence that oxidative stress and nitrosative stress (another form of oxidative-antioxidative imbalance) are involved in the pathophysiology of migraine26,30. Environmental factors that increase the probability of migraine attacks (so-called migraine triggers) such as alcohol, air pollutants and cigarette smoke commonly have a capacity to induce oxidative stress18. Moreover, studies have shown that Flunarizine, vitamin C, vitamin E, and coenzyme Q10, which are used in migraine prevention, decrease oxidative stress via their ROS scavenger properties24,31,32. These studies and our study show that irrespectively of environmental and genetic factors, migraine attack is associated with the occurrence of oxidative stress, however, it is not known how oxidative stress triggers a migraine attack. It is suggested that several oxidative stress-related mechanisms contribute to migraine pathogenesis. So far, two major theories of vascular and neurovascular origins have been suggested in migraine pathophysiology. Based on the vascular theory that was first presented by Graham and Wolff, the dilation of cerebral vessels could be a cause of migraine headache33. One major mechanism of vasodilation is the activation of ATP-sensitive and calcium-activated potassium channels in cerebral vascular muscle34. There is evidence that ROS, such as hydrogen peroxide and peroxynitrite (a product of the reaction of nitric oxide with superoxide), reversibly causes cerebral vasodilation by activating ATP-sensitive potassium channels most probably through an oxidant mechanism; whereas superoxide dilates cerebral vessels by opening calcium-activated potassium channels35,36. In another study, it was shown that the effect of ROS on ATP-sensitive potassium channels of the cells in the cerebral arterioles is blocked by antioxidants37.
The neurovascular theory has integrated both vascular and neurological events occurring in migraine. Base on this theory, migraine headache is associated with the inflammation of the meninges, particularly the dura38. During migraine attack, the trigeminal sensory nerves innervating dural blood vessels become extra sensitive and release vasoactive modulatory neuropeptides such as the neurokinins, substance P and calcitonin gene-related peptides (CGRP), which may play a role in meningeal inflammation and migraine pain38,39,40. This theory also implicates the phenomenon of cortical spreading depression as an underlying mechanism of migraine aura40. There is evidence that increased oxidative stress contributes to various pathological states including trigeminovascular inflammation, which occurs during migraine attacks41,42. It has been shown that ROS, including H2O2, can activate sensory neurons directly and/or indirectly by releasing endogenous CGRP as a mediator of migraine headache43.
Conclusions
This study showed that oxidative-antioxidative balance shifts towards oxidative status during migraine attack. An increase in oxidant levels, either with or without the depletion of antioxidant activity, may contribute to migraine pathogenesis by affecting cerebral blood vessel diameter and by activating trigeminal sensory nerves innervating dural blood vessels.
Abbreviations
PC: Protein carbonyl
MDA: Malondialdehyde
TOS: Total oxidants status
T-SH: Total thiols
GSH: Glutathione
TAC: Aantioxidant capacity
OSI: Oxidative stress index
ROS: Reactive oxygen species
SSA: Sulfosalicylic acid
ELISA: Enzyme-linked immunosorbent assay
ABTS: 2, 2-azinobis 3-ethylbenzothiazoline-6-sulfonate
CGRP: Calcitonin gene-related peptide
Competing Interests
Authors have no conflict of interest.
Authors' Contributions
Alireza Khosravi: Design of the study, acquisition of data, and writing of the article.
Abdoreza Ghoreishi: Collection of data and revision of the article.
Alireza Nakhaee: Analysis and interpretation of data.
Masoud Sadeghi: Revision of the article and final approval of the version to be published.
Zahra Arefpoor: Revision of the article.
References
-
Fattahian
R.,
Sadeghi
M.,
Mozaffari
H.R.,
The association between allergic rhinitis with migraine: A systematic review and meta-analysis study. Med Sci.
2018;
22
:
225-31
.
-
Society
Headache Classification Subcommittee of the International Headache,
The International Classification of Headache Disorders: 2nd edition. Cephalalgia.
2004;
24 Suppl 1
:
9-160
.
-
Wessman
M.,
Terwindt
G.M.,
Kaunisto
M.A.,
Palotie
A.,
Ophoff
R.A.,
Migraine: a complex genetic disorder. Lancet Neurol.
2007;
6
(6)
:
521-32
.
View Article PubMed Google Scholar -
M. Sparaco,
Feleppa
M.,
Lipton
R.,
A. Rapoport,
Bigal
M.,
Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia.
2006 ;
26
(4)
:
361-72
.
View Article PubMed Google Scholar -
L. Edvinsson,
Neuronal signal substances as biomarkers of migraine. Headache.
2006 ;
46
(7)
:
1088-94
.
View Article PubMed Google Scholar -
B. Uttara,
A.V. Singh,
P. Zamboni,
R.T. Mahajan,
Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol.
2009 ;
7
(1)
:
65-74
.
View Article PubMed Google Scholar -
J. Limon-Pacheco,
M.E. Gonsebatt,
The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res.
2009 ;
674
(1-2)
:
137-47
.
View Article PubMed Google Scholar -
Y. Eren,
E. Dirik,
S. Neselioglu,
O. Erel,
Oxidative stress and decreased thiol level in patients with migraine: cross-sectional study. Acta Neurol Belg.
2015 ;
115
(4)
:
643-9
.
View Article PubMed Google Scholar -
Pham-Huy
L.A.,
He
H.,
Pham-Huy
C.,
Free radicals, antioxidants in disease and health. Int J Biomed Sci.
2008;
4
(2)
:
89-96
.
PubMed Google Scholar -
Hu
M.L.,
Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol.
1994;
233
:
380-5
.
View Article PubMed Google Scholar -
D. Koracevic,
G. Koracevic,
V. Djordjevic,
S. Andrejevic,
V. Cosic,
Method for the measurement of antioxidant activity in human fluids. J Clin Pathol.
2001 ;
54
(5)
:
356-61
.
PubMed Google Scholar -
Cheeseman
K.H.,
Slater
T.F.,
An introduction to free radical biochemistry. Br Med Bull.
1993;
49
(3)
:
481-93
.
View Article PubMed Google Scholar -
T. Finkel,
N.J. Holbrook,
Oxidants, oxidative stress and the biology of ageing. Nature.
2000 ;
408
(6809)
:
239-47
.
View Article PubMed Google Scholar -
I. Dalle-Donne,
R. Rossi,
D. Giustarini,
A. Milzani,
R. Colombo,
Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta.
2003 ;
329
(1-2)
:
23-38
.
PubMed Google Scholar -
Boćkowski
L.,
Sobaniec
W.,
Ku\lak
W.,
Smigielska-Kuzia
J.,
Serum and intraerythrocyte antioxidant enzymes and lipid peroxides in children with migraine. Pharmacol Rep.
2008;
60
(4)
:
542-8
.
PubMed Google Scholar -
J
J.M. Mates,
C. Pérez-Gómez,
I.N. De Castro,
Antioxidant enzymes and human diseases. Clin Biochem.
1999 ;
32
(8)
:
595-603
.
-
M. Valko,
D. Leibfritz,
J. Moncol,
M.T. Cronin,
M. Mazur,
J. Telser,
Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol.
2007;
39
(1)
:
44-84
.
View Article PubMed Google Scholar -
Borkum
J.M.,
Migraine Triggers and Oxidative Stress: A Narrative Review and Synthesis. Headache.
2016;
56
(1)
:
12-35
.
View Article PubMed Google Scholar -
O. Erel,
A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem.
2004 ;
37
(2)
:
112-9
.
PubMed Google Scholar -
O. Erel,
A new automated colorimetric method for measuring total oxidant status. Clin Biochem.
2005 ;
38
(12)
:
1103-11
.
View Article PubMed Google Scholar -
A. Nakhaee,
F. Shahabizadeh,
M. Erfani,
Protein and lipid oxidative damage in healthy students during and after exam stress. Physiol Behav.
2013 ;
13
(118)
:
118-21
.
View Article PubMed Google Scholar -
M.S. Moron,
J.W. Depierre,
B. Mannervik,
Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta.
1979 ;
582
(1)
:
67-78
.
PubMed Google Scholar -
M. Mihara,
M. Uchiyama,
Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem.
1978;
86
(1)
:
271-8
.
PubMed Google Scholar -
I. Ciancarelli,
M. Tozzi‐Ciancarelli,
C. Di Massimo,
C. Marini,
A. Carolei,
Flunarizine effects on oxidative stress in migraine patients. Cephalalgia.
2004 ;
24
(7)
:
528-32
.
View Article PubMed Google Scholar -
D. Tuncel,
F.I. Tolun,
M. Gokce,
S. İmrek,
H. Ekerbiçer,
Oxidative stress in migraine with and without aura. Biol Trace Elem Res.
2008;
126
(1-3)
:
92-7
.
View Article PubMed Google Scholar -
G. Yilmaz,
H. Sürer,
L.E. Inan,
Ö. Coskun,
D. Yücel,
Increased nitrosative and oxidative stress in platelets of migraine patients. Tohoku J Exp Med.
2007 ;
211
(1)
:
23-30
.
PubMed Google Scholar -
Alp
R.,
Selek
S.,
Alp
S.I.,
Taşkin
A.,
Koçyiğit
A.,
Oxidative and antioxidative balance in patients of migraine. Eur Rev Med Pharmacol Sci.
2010;
14
(10)
:
877-82
.
PubMed Google Scholar -
R. Gupta,
R. Pathak,
M.S. Bhatia,
B.D. Banerjee,
Comparison of oxidative stress among migraineurs, tension-type headache subjects, and a control group. Ann Indian Acad Neurol.
2009 ;
12
(3)
:
167-172
.
View Article PubMed Google Scholar -
C. Bernecker,
C. Ragginer,
G. Fauler,
R. Horejsi,
R. Möller,
S. Zelzer,
Oxidative stress is associated with migraine and migraine-related metabolic risk in females. Eur J Neurol.
2011 ;
18
(10)
:
1233-9
.
View Article PubMed Google Scholar -
Gruber
H.J.,
C. Bernecker,
A. Lechner,
S. Weiss,
M. Wallner-Blazek,
A. Meinitzer,
Increased nitric oxide stress is associated with migraine. Cephalalgia.
2010 ;
30
(4)
:
486-92
.
View Article PubMed Google Scholar -
S. Chayasirisobhon,
Use of a pine bark extract and antioxidant vitamin combination product as therapy for migraine in patients refractory to pharmacologic medication. Headache.
2006 ;
46
(5)
:
788-93
.
View Article PubMed Google Scholar -
C. Gaul,
H.C. Diener,
U. Danesch,
Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain.
2015;
16
(32)
.
View Article PubMed Google Scholar -
J.R. Graham,
H.G. Wolff,
Mechanism of migraine headache and action of ergotamine tartrate. Arch Neurol Psychiatry.
1938;
39
(4)
:
737-763
.
View Article PubMed Google Scholar -
Kitazono
T.,
Faraci
F.M.,
Taguchi
H.,
Heistad
D.D.,
Role of potassium channels in cerebral blood vessels. Stroke.
1995;
26
(9)
:
1713-23
.
View Article PubMed Google Scholar -
F.M. Faraci,
Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol (1985).
2006 ;
100
(2)
:
739-43
.
View Article PubMed Google Scholar -
E.P. Wei,
H.A. Kontos,
Beckman
J.S.,
Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol.
1996 ;
271
(3 Pt 2)
:
H1262-6
.
View Article PubMed Google Scholar -
Wei
E.P.,
Kontos
H.A.,
Beckman
J.S.,
Antioxidants inhibit ATP-sensitive potassium channels in cerebral arterioles. Stroke.
1998;
29
(4)
:
817-22
.
View Article PubMed Google Scholar -
Moskowitz
M.A.,
Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology.
1993;
43
(6)
:
S16-20
.
PubMed Google Scholar -
D.K. Arulmozhi,
A. Veeranjaneyulu,
S.L. Bodhankar,
Migraine: current concepts and emerging therapies. Vascul Pharmacol.
2005 ;
43
(3)
:
176-87
.
View Article PubMed Google Scholar -
F. Galletti,
L.M. Cupini,
I. Corbelli,
P. Calabresi,
P. Sarchielli,
Pathophysiological basis of migraine prophylaxis. Prog Neurobiol.
2009 ;
89
(2)
:
176-92
.
View Article PubMed Google Scholar -
D.A. Andersson,
C. Gentry,
S. Moss,
S. Bevan,
Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci.
2008 ;
28
(10)
:
2485-94
.
View Article PubMed Google Scholar -
J.E. Keeble,
J.V. Bodkin,
L. Liang,
R. Wodarski,
M. Davies,
E.S. Fernandes,
Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain.
2009;
141
(1-2)
:
135-42
.
View Article PubMed Google Scholar -
A. Shatillo,
K. Koroleva,
R. Giniatullina,
N. Naumenko,
A.A. Slastnikova,
R.R. Aliev,
Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience.
2013 ;
253
:
341-9
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 2 (2019)
Page No.: 2996-3002
Published on: 2019-02-24
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8084 times
- Download PDF downloaded - 2084 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress


