Abstract
Introduction: The aim of this study was to investigate whether the circulating level of sST2 would predict adverse LV remodeling in STEMI patients with TIMI III flow through the myocardial infarctrelated coronary artery six months after intervention.
Methods: The study retrospectively included 65 patients with STEMI and TIMI-III flow after primary or facilitated percutaneous coronary intervention (PCI). These patients were admitted to the intensive care unit of L.T. Malaya Therapy National Institute between August 2016 and July 2018. Primary PCI with bare-metal stent implantation was performed in 33 patients, and 32 patients were previously treated with primary thrombolysis followed by PCI within 12 hours after initial STEMI confirmation. Angiographic, clinical, and biochemical parameters were evaluated. B-mode, Tissue Doppler, Strain Echocardiography, and blood sampling for biomarker assays were performed at admission, at discharge from the hospital, and at six months after STEMI.
Results: Late adverse LV remodeling is defined as an increase of LV end-diastolic volume (EDV) six months post STEMI (first cohort, n=29), while other patients (second cohort, n=36) did not demonstrate a decreasing trend of LV EDV, or they had never revealed any decrease of this parameter. There was a significant difference between the two cohorts in the serum level of sST2 at discharge, while the levels of natriuretic peptides, troponin I were similar (P=0.24). Indeed, the circulating level of sST2 in the first cohort was higher than that of the second cohort (59.72 ng/mL; 95% confidence interval [CI] = 36.99 ng/mL -139.53 ng/mL versus 44.75 ng/mL; 95%CI =28.25 ng/mL -77.32 ng/mL, P=0.039, respectively). ROC-analyses showed that the best balanced cut-off point for sST2 to predict adverse remodeling at 6 months post PCI was 35 ng/mL (AUC=0.672 95% C 0.523-0.799; P=0.0344 sensitivity = 46.7% and specificity = 85.7%).
Conclusions: We showed that the circulating level of sST2 measured at discharge in acute STEMI patients intervented by PCI could predict late adverse LV remodeling six months post PCI. These findings offer a new biomarker to stratify patients with successful coronary re-vascularization at risk of HF.
Introduction
Recent clinical studies have shown that primary and facilitated percutaneous coronary interventions (PCI) in patients with acute ST-segment elevation myocardial infarction (STEMI) are effective to prevent early cardiac remodeling and to improve survival in short-term and long-term perspective 12. Indeed, observational and clinical studies revealed that the angiographic benefit of TIMI III significantly associated with a lower incidence of recurrent ischemia and newly atherothrombotic events. This procedure also requires fewer re-vascularization procedures 30 days post-PCI 34. Current ESC clinical guideline emphasizes that earlier PCI in patients with acute STEMI correlates with a significant reduction in the composite outcome of death, heart failure, or stroke after one year compared to patients with delayed PCI 5. However, improvement in coronary reperfusion in acute STEMI patients after early-PCI is not able to completely prevent late (four months post-PCI) adverse cardiac left ventricular (LV) remodeling associating with a steady increase of LV end-diastolic volume, shaping of LV sphericity, and decline LV pump function 6. Importantly, late LV adverse cardiac remodeling is not a bias toward early primary PCI or delayed primary/facilitated PCI and thrombolysis, which needs to be further investigated.
Late adverse cardiac remodeling after STEMI is a result of a combination of different factors including loss of cardiomyocyte mass, sarcomere rearrangement, extracellular matrix deposition, inflammatory signaling, and immune cell activation. These complications lead to a progressive increase in systolic and diastolic LV volumes 7. Both early (< 4 months) and late adverse cardiac remodelings (4-24 months) are the most common causes of heart failure (HF) and poor long-term prognosis 8. Even though early functionally restored revascularization effectively prevents alterations in ventricular architecture and thereby stop developing early adverse cardiac remodeling, there is no compelling evidence that late adverse cardiac remodeling could be effectively prevented by restoring coronary blood flow beyond the use of peri-procedural and post-procedural drugs (i.e., statins, double anti-platelet therapy, abciximab, tirofiban, ACE inhibitors, etc.) 9101112. In this context, biomarkers would be a useful tool to indicate whether candidates for PCI are at high risk of poor clinical outcomes relating to late adverse cardiac remodeling and whether they should be treated by an alternative method.
Biomarkers reflect various patho-physiological aspects of spherical LV transformation that relate to myocardial stress due to persisted ischemia, fibrosis, and inflammation. Hence, these biomarkers are helpful to improve risk stratification, to personalize medical care to prevent HF, and to adjust treatments after STEMI 13. In this context, suppression of tumorigenicity 2 (ST2) appears to be a promising biomarker.
ST2 is a member of the superfamily of interleukin [IL]-1-dependent cytokines and exists as transmembrane (ST2L) or soluble (sST2) protein. Cardiac myocytes and fibroblasts are able to express both ST2L and sST2 in response to triggers (mechanical stretching, pro-inflammatory activation, and Th-2-dependent reactions) 14. Soluble ST2 binds to circulating IL-33. This interaction prevents the cardio-protective properties of IL-33 and induces myocardial fibrosis, inflammation, apoptosis, and hypertrophy 15. The serum level of sST2 is elevated in half of the patients with STEMI and highly correlates with adverse cardiac events and a higher risk of newly diagnosed HF, cardiovascular death, and recurrent admission to hospital independently of other prognostic indicators 16. Whether serum sST2 could be used to prognose cardiac remodeling after STEMI is not fully clear. This study aimed to investigate the role of the circulating level of sST2 in predicting adverse LV remodeling in patients with the first STEMI six months post-PCI.
Methods
Patients’ population
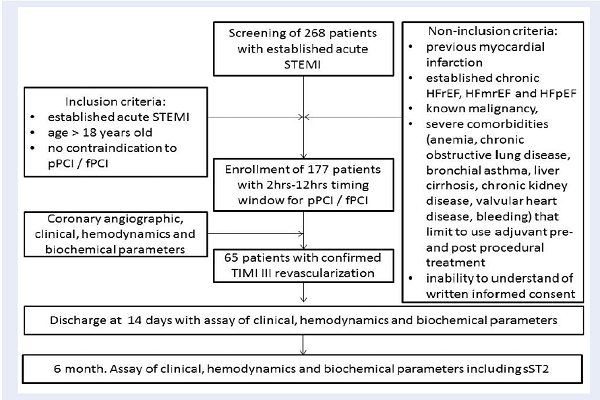
A total of 268 patients with confirmed acute STEMI were screened for participation in the study. Flowchart of the study design is shown in Figure 1. From the entire population of STEMI (n=268) and according to the inclusion and non-inclusion criteria, 177 individuals who were admitted to intensive care unit of GI “L.T.Malaya TNI NAMSU” with acute STEMI within 2-12 hours of symptoms onset in between August 2016 and July 2018 were enrolled into the study. STEMI was diagnosed according to the ECS Guidelines (2017) 5. Inclusion criteria included confirmed STEMI, age > 18 years old, and lack of contraindication to PCI. Non-inclusion criteria included previous myocardial infarction, established chronic HFrEF, HFmrEF, and HFpEF, known malignancy, severe comorbidities (anemia, chronic obstructive lung disease, bronchial asthma, liver cirrhosis, chronic kidney disease, valvular heart disease, bleeding), inability to understand of written informed consent. The final study cohort retrospectively included 65 patients with confirmed STEMI after primary or facilitated PCI with successful re-vascularization of TIMI-III. Primary PCI with bare-metal stent (COMMANDER, “Alvimedica”, Turkey) implantation was performed in 33 patients, and 32 patients were previously treated with primary thrombolysis (tenecteplase, alteplase) before admission, which was followed by PCI within six to twelve hours after the initial STEMI confirmation. Thrombolysis was done with tenecteplase (Metalise, Boehringer Ingelheim Pharma, Germany), depending on patients weight and was not more than 50 mg iv bolus. Alteplase (Actilyse, Boehringer Ingelheim Pharma, Germany) 100mg was infused intravenously for two hours. All investigated patients received adjuvant treatment according to the current ESC recommendations 5.
Ethical declaration
The study complied with the Declaration of Helsinki and was approved by the local ethics committee (Protocol №8, 29.08.2016). All patients signed informed consent to participate in the study.
Coronary angiography
Conventional coronary angiography was performed using Digital X-Ray system “Integris Allura” (Philips Healthcare, Best, The Netherlands) and managed by radial or femoral vascular access. Coronary arteries were visualized with two-to-three orthogonal projections. In this study, the contrast “Ultravist-370” (Baier Pharma GmbH, Germany) and automatic contrast injector were used. The contrast amount used in coronary angiography in each injection was 8 – 10 mL at 4 mL/s for the left coronary artery and 6 mL at 3 mL/s for the right coronary artery (radiation exposure 20 to 35 mGycm). The number of views obtained was decided by the operator depending on coronary anatomy. The coronary arteries were divided into segments according to the American Heart Association classification 17. TIMI score was used to validate prognostic capacity after STEMI 18.
SYNTAX score determination
SYNTAX score (SS) was used to assess the severity of coronary atherosclerotic lesions, and it was calculated by an experienced interventional cardiologist. SS was determined for all coronary lesions >50% diameter stenosis in a vessel >1.5 mm based on SS calculator (www.syntaxscore.com) 19. The severity of coronary atherosclerotic lesions was determined as high (SS score >32 points), average (22 points < SS score ≤32 points), and low (SS score ≤22 points).
Determination of risk factors and comorbidities
Hypercholesterolemia (HCE) was diagnosed if the total cholesterol (TC) level was above 5.2 mmol/L, and/or the low-density lipoprotein cholesterol (LDL) level was above 3.0 mmol/L, and/or the level of triglycerides (ТG) was above 1.7 mmol/L according to the European Cardiology Society dyslipidemia guideline, 2016 20.
Hypertension was diagnosed if the systolic blood pressure (SBP) was >140 mm Hg, and/or the diastolic blood pressure (DBP) >90 mm Hg according to the European guideline on diagnostics and treatment of arterial hypertension, 2018 21.
Type 2 diabetes mellitus was determined according to the new ADA statement (2017) 22.
Echo and Doppler examination
Echo-CG was performed on “Medison Sono Ace X6” device (Korea) by using a phase probe with an ultrasound frequency of 3.5 MHz at discharge and at six months post-PCI. Left ventricular end diastolic volume (LV EDV), left ventricular end systolic volume (LV ESV), left ventricular end diastolic and end systolic diameters (LV EDD, LV ESD), left ventricular ejection fraction (LVEF) were measured according to the conventional method 23. LV global longitudinal strain (%) was measured at the baseline and six months per protocol 23. Left ventricular myocardial mass (LVMM) was calculated automatically according to the current recommendation 24.
Determination of late adverse cardiac remodeling
Late adverse cardiac remodeling was defined as increased LVEDV (>10% from baseline) and/or LVESV (>10% from baseline) six months after acute STEMI managed by PCI 25.
Calculation of glomerular filtration rate
Glomerular filtration rate (GFR) was calculated by CKD-EPI formula 26.
Blood samples
Blood samples were drawn immediately before PCI and six months post-PCI. Blood samples were centrifuged, serum was isolated within 30 minutes of sample acquisition, then they were stored in plastic tubes and frozen at -700C until being shipped to the laboratory of immune-chemical and molecular-genetic researches of GI “L.T.Malaya TNI NAMSU”.
Troponin I (Tn I) level was measured by chemoluminescent immunoassay (Humalyzer 2000, HUMAN GmbH, Germany). The average of Tn I level was 0.5-50 ng/mL.
Total creatine kinase (CK) and CK MB-fraction (CK-MB) were analyzed by immunoinhibition method on the quantitative immunoassay analyzer Humalyser 2000 (HUMAN GmbH, Germany) according to the manufacturers’ recommendations.
Total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides (TG) were measured by direct enzymatic method (Roche P800 analyzer, Basel, Switzerland). The intra-assay and inter-assay coefficients of variation were <5%.
Fasting glucose level was measured by double-antibody sandwich immunoassay (Elecsys 1010 analyzer, F. Hoffmann-La Roche Diagnostics, Mannheim, Germany). The intra-assay and inter-assay coefficients of variation were <5%.
N-terminal fragment of brain natriuretic peptide (NT-proBNP) was measured by a commercially available standard kit (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). The average of NT-proBNP level was 10-12000 pg/mL.
The circulating level of sST2 was measured by specific enzyme-linked immunosorbent assay (Presage ST2 Assay, Critical Diagnostics, USA). The average of the sST2 circulating level was 0-29 ng/mL.
| Variables | Entire group (n=65) | Cohort 1 (n=29) | Cohort 2 (n=36) | Р value between both cohorts |
| Demographic, risk factors and comorbidities | ||||
| Age (years) | 58.71±11.04 | 56.25±7.78 | 59.15±7.52 | 0.133 |
| Male (%) | 54 (83.1) | 23 (79.3) | 31 (86.1) | 0.693 |
| Hypertension (%) | 42 (64.6) | 27 (75.0) | 15 (51.7) | 0.091 |
| T2DM (%) | 9 (13.8) | 3 (10.3) | 6 (16.7) | 0.710 |
| BMI, kg/m 2 | 29.93±5.24 | 30.62±6.43 | 29.66±4.57 | 0.485 |
| eGFR, mL/min 1.73 m 2 | 71.6 [66.2 – 85.4] | 70.2 [65.3 – 83.9] | 73.1 [65.0 – 86.7] | 0.780 |
| Hypercholesterolemia (%) | 38 (58.5) | 16 (55.2) | 22 (61.1) | 0.629 |
| Current smoker (%) | 24 (36.9) | 10 (34.5) | 14 (38.9) | 0.714 |
| STEMI localization | ||||
| Anterior, n (%) | 38 (58.5) | 21 (62.1) | 17 (45.3) | 0.041 |
| Posterior | 27 (41.5) | 8 (27.6) | 19 (52.8) | |
| TIMI risk score | 6 [4-7] | 6 [5-7] | 6 [4-7] | 0.98 |
| Mean SYNTAX score | 25.7±6.9 | 27.5±7.4 | 21.6±4.8 | 0.443 |
| >32 points, n (%) | 28 (43.1) | 12 (41.4) | 16 (44.4) | 0.924 |
| 22 - 32 points, n (%) | 29 (44.6) | 12 (41.4) | 17 (47.2) | 0.886 |
| ≤22 points, n (%) | 8 (12.3) | 5 (17.2) | 3 (8.3) | 0.126 |
| Killip class | ||||
| І-ІІ class, n (%) | 56 (86.2) | 27 (93.1) | 29 (80.6) | 0.145 |
| IIІ-ІV class, n (%) | 9 (13.8) | 2 (6.9) | 7 (19.4) | |
| Number of damaged coronary vessels | ||||
| One, n (%) | 12 (18.5) | 3 (10.3) | 9 (25.0) | 0.130 |
| Two, n (%) | 10 (15.4) | 6 (20.7) | 4 (11.1) | 0.473 |
| Three, n (%) | 10 (15.4) | 5 (17.2) | 5 (13.9) | 0.979 |
| Culprit coronary artery | ||||
| Left anterior descending, n (%) | 22 (33.8) | 9 (31.0) | 13 (36.1) | 0.122 |
| Right coronary artery, n (%) | 25 (38.5) | 12 (41.4) | 13 (36.1) | 0.446 |
| Circumflex coronary artery, n (%) | 13 (20.0) | 6 (20.7) | 7 (19.4) | 0.886 |
| Left main, n (%) | 5 (7.7) | 2 (6.9) | 3 (8.3) | 0.724 |
| Biomarkers of necrosis and myocardial stress | ||||
| Peak TnI, ng/mL | 25 [11-33] | 28 [10-39] | 23 [14-31] | 0.446 |
| Peak CK-MB, U/L | 1226 [660–2523] | 1254 [820–2462] | 1213 [540–2672] | 0.661 |
| NT-proBNP, pg/mL | 247 [118 - 388] | 253 [121 - 375] | 241 [105 - 344] | 0.688 |
| Lipids | ||||
| Total cholesterol, mmol / L | 6.24 [4.97-8.11] | 6.30 [5.10-8.03] | 6.19 [4.92-7.80] | 0.788 |
| HDL-cholesterol, mmol / L | 0.98 [0.92-1.06] | 0.94 [0.91-1.02] | 0.99 [0.92-1.09] | 0.448 |
| LDL-cholesterol, mmol / L | 3.18 [2.90-3.50] | 3.23 [2.98-3.80] | 3.14 [2.70-3.50] | 0.412 |
| Medication at discharge | ||||
| ACE inhibitor or ARB, n (%) | 61 (93.8) | 27 (93.1) | 34 (94.4) | 0.921 |
| Beta-blockers, n (%) | 65 (100) | 29 (100) | 36 (100) | 1.0 |
| Statin, n (%) | 65 (100) | 29 (100) | 36 (100) | 1.0 |
| Aspirin, n (%) | 63 (96.9) | 29 (100) | 34 (94.4) | 0.921 |
| Thienopyridines, n (%) | 62 (95.4) | 26 (89.7) | 36 (100) | 0.786 |
| MCRAs, n (%) | 48 (73.8) | 20 (68.9) | 28 (77.8) | 0.848 |
Statistics
Statistical analyses were performed using SPSS v. 23 (USA). Continuous variables are presented as mean ± standard deviation when normally distributed, or median and interquartile range if otherwise. Categorical variables are presented as frequencies and percentages. Mann-Whitney and Wald-Wolfowitz criteria were used for intergroup differences and quantitative values. The qualitative variables are expressed as percentages, and were analyzed by the χ2 and exact Fisher tests. Receiver operating characteristic (ROC) curve was performed to detect well-balanced cut-off of sST2. Correlations between the level of sST2 and other variables were analyzed by univariate linear regression analysis. We performed univariate and multiple variate log-regression analysis to determine factors that could predict late adverse cardiac remodeling six months post-PCI. We calculated the beta coefficient, standard errors (SE), odds ratio (OR), 95% confidence interval (CI) for each factor. Factor, for which P values were calculated as >0.5 were not included in the multiple variate log-regression analysis. All differences were considered statistically significant with two-tailed <0.05.
Results
The total number of STEMI patients was 65 (83.1% male with the mean of age of 58.71 years). Table 1 reported the basic characteristics of STEMI patients included in the study. Hypertension was determined in 64.6%; type 2 diabetes mellitus was referred in 13.8%; hypercholesterolemia was found in 58.5% of the total number of patients. About 37% of patients from the entire group were active smokers. The values of BMI and eGFR for both cohorts were similar. There were no significant differences between the two cohorts in demographic and medical history.
The entire group consists of 58.5% of patients with established anterior acute STEMI and 41.4% of patients with posterior localization of STEMI. However, there was a significant prevalence of anterior localization of acute STEMI in the first cohort and posterior localization of acute STEMI in the second cohort (P=0.041). TIMI score and SYNTAX scores were 6 points [4-7 points] and 25.7±6.9 respectively for the entire patient population. There were no significant differences between the two cohorts in TIMI score and SYNTAX score. Additionally, there was no significant difference in the acute HF Killip score between both cohorts at the baseline (P=0.145). The analysis of coronary angiograms revealed one artery disease in 18.5% of the entire group of STEMI patients, as well as two and three coronary artery damages in 15.4% and 15.4% of the entire group respectively. The significant differences between the two cohorts in the number of damaged coronary vessels were not determined. Damaged left anterior descending artery was found in 33.8% patients, damaged right coronary artery was detected in 38.5% cases, damaged circumflex coronary artery was found in 20.0% cases, and damaged left main artery was verified in 7.7% patients. There was no difference between the two cohorts in the number of culprit coronary arteries. Biomarkers of necrosis (peak TnI and peak CK-MB) and myocardial stress (NT-proBNP) were elevated in the entire group, and the differences between the levels of these biomarkers in the two cohorts at the baseline were not significant.
All patients were treated with 80 mg of atorvastatin or 40 mg of rosuvastatin, dual antiplatelet therapy (aspirin + clopidogrel or ticagrelor per contemporary protocol) before the procedure. After PCI, patients were stabilized with ACE inhibitors or ARBs, beta-blockers, antiplatelets (aspirin, clopidogrel or ticagrelor), mineralocorticoid receptor antagonists (eplerenone or spironolactone) and statins in recommended doses. There was no difference in the treatment scheme between two patient cohorts.
Table 2 reported hemodynamics in STEMI patients at the baseline and six months post-PCI. We did not find any significant difference between the two cohorts in hemodynamics at the baseline. At six months post-PCI, systolic and diastolic blood pressure, heart rates, LVEF and LVMM were similar between the two cohorts. Additionally, LVESV and LVEDV were higher in the first cohort in comparison to the second cohort, while values of LVEF did not differ between the two cohorts six months post-PCI. In contrast, LV global longitudinal strain was significantly lower in the first cohort compared to the second cohort.
The average levels of sST2 in the entire group and the two cohorts are reported in Figure 2. The first cohort had a higher circulating level of sST2 compared to the second cohort (Р=0.039).
The univariate regression analysis showed that the level of sST2 at discharge was significantly correlated with T2DM (r=0.41; P=0.001), LV global longitudinal strain six months post-PCI (r=-0.43; P=0.001), LVEF six months post-PCI (r=-0.42; Р=0,006), HDL-cholesterol at discharge (r=-0.33; Р=0,007), SYNTAX score at admission (r=0.42; P=0.001), acute HF Killip score II-IV at admission (r=0.44; P=0.001), peak TnI at admission (r=0.33; P=0.001), number of damaged coronary vessels at admission (r=0.32; P=0.002), left main culprit lesion at admission (r=0.36; P=0.002), and NT-proBNP (r=0.31; P=0.002).
| Variables | Entire group (n=65) | Cohort 1 (n=29) | Cohort 2 (n=36) | Р value between both cohorts |
| At baseline | ||||
| SBP, mm Hg | 135.31±29.87 | 127.68±23.88 | 135.67±30.24 | 0.250 |
| DBP, mm Hg | 80.00±13.37 | 77.79±12.80 | 81.23±11.56 | 0.260 |
| HR, per 1 min. | 76.63±16.64 | 77.50±15.49 | 67.68±12.55 | 0.07 |
| LV ESV, mL | 65.78±32.26 | 70.33±28.92 | 62.60±21.52 | 0.06 |
| LV EDV, mL | 137.56±38.21 | 138.00±38.16 | 136.00±34.04 | 0.17 |
| LV MM, g | 229.60±51.43 | 240.30±30.38 | 232.33±64.66 | 0.543 |
| LV global longitudinal strain (%) | −14.1 ± 2.0 | −13.9 ± 1.8 | −14.5 ± 1.9 | 0.664 |
| LV EF, % | 53.10±10.33 | 52.79±8.22 | 53.88±9.44 | 0.473 |
| At 6 month | ||||
| SBP, mm Hg | 139.12±30.6 | 136.62±26.34 | 134.46±18.7 | 0.630 |
| DBP, mm Hg | 83.02±15.52 | 77.69±11.66 | 81.62±10.48 | 0.429 |
| HR, per 1 min | 68.95±10.3 | 69.78±12.51 | 68.83±12.18 | 0.868 |
| LVESV, mL | 70.10±24.3 | 84.0±17.62 | 66.60±15.44 | 0.049 |
| Δ LVESV, mL | 6.3±2.30 | 16.7±3.90 | 5.4±2.60 | 0.001 |
| LVEDV, mL | 146.00±27.37 | 161.00±14.92 | 135.3±13.4 | 0.048 |
| Δ LVEDV, mL | 5.9±1.15 | 14.3±2,40 | -0.5±0.11 | 0.001 |
| LVMM, g | 255.69±89.41 | 261.51±35.24 | 218.98±60.44 | 0.057 |
| LV global longitudinal strain (%) | −13.2 ± 2.0 | −11.4 ± 1.2 | −14.7 ± 1.6 | 0.048 |
| LVEF, % | 53.13±5.70 | 47.78±5.31 | 52.80±4.38 | 0.05 |
We used ROC curve analysis to determine the best-balanced cut-off point for the sST2 level with optimal prognostic significance for late adverse cardiac remodeling. We found that the sST2 level equals to 35 ng/mL or more at discharge accurately predicted adverse cardiac remodeling in STEMI six months post-PCI (Area Under Curve [AUC] =0.672 95% CІ 0.523-0.799; Р=0.0344 sensitivity 46,7%; specificity 85.7%) (Figure 3).
We performed a univariate and multiple variate log-regression analysis to determine factors that could predict late adverse cardiac remodeling in patients with STEMI managed by PCI (Table 3). The analysis showed that sST2 > 35 ng/mL was an independent predictor of late adverse cardiac remodeling. Other significant variables which emerged as independent predictors of late adverse cardiac remodeling included left main culprit lesion and multiple damaged coronary vessels at admission.
| Variables | Univariate log-regression analysis | Multiple variate log-regression analysis | ||||||||
| β | SE | OR | 95% CI | β | SE | OR | 95% CI | P | ||
| LVEF at discharge | 0.06 | 0.049 | 1.01 | 1.00-1.03 | 0,17 | - | - | - | - | - |
| T2DM (present vs absent) | 0.83 | 0.14 | 1.02 | 1.01-1.04 | 0.044 | 0.75 | 0.11 | 1.01 | 1.0-1.02 | 0.06 |
| sST2 (> 35 ng/mL vs < 35 ng/mL) | 1.49 | 0.25 | 1.16 | 1.09-1.26 | 0.001 | 1.28 | 0.11 | 1.12 | 1.1-1.16 | 0.002 |
| LV global longitudinal strain at discharge | 0.73 | 0.20 | 1.03 | 1.01-1.06 | 0.046 | 0.80 | 0.19 | 1.02 | 1.0-1.04 | 0.052 |
| SYNTAX score at admission | 0.99 | 0.19 | 1.08 | 1.03-1.15 | 0.001 | 1.26 | 0.15 | 1.05 | 1.00-1.8 | 0.14 |
| HDL-C at discharge | -0.43 | 0.12 | 1.01 | 0.98-1.03 | 0.67 | - | - | - | - | - |
| NT-proBNP at discharge | 1.15 | 0.21 | 1.03 | 1.01-1.04 | 0.046 | 1.12 | 0.12 | 1.03 | 1.00-1.05 | 0.054 |
| Acute HF Killip score at admission (≥III versus ≤II) | 0.95 | 0,05 | 1.03 | 1.01-1.06 | 0.048 | 1.03 | 0.03 | 1.02 | 1.00-1.04 | 0.054 |
| Left main culprit lesion at admission | 1.88 | 0.34 | 1.07 | 1.02-1.12 | 0.001 | 1.22 | 0.32 | 1.08 | 1.03-1.12 | 0.001 |
| Peak TnI at admission | 1.06 | 0.11 | 1.02 | 1.01-1.04 | 0.05 | - | - | - | - | - |
| Number of damaged coronary vessels at admission (3 versus <3) | 1.17 | 0.38 | 1.04 | 1.03-1.05 | 0.026 | 1.10 | 0.25 | 1.03 | 1.01-1.05 | 0.048 |
Discussion
The results of the study indicated that the elevated level of sST2 (> 35 ng/mL) could independently predict six-month adverse cardiac remodeling in acute STEMI patients who were effectively managed by primary or facilitated PCI. Additional important factors contributing to LV dilatation, altered LV diastolic function, and declined LVEF were multiple damaged coronary arteries and left main stenotic lesion at admission. In fact, complete early re-vascularization in acute STEMI is believed to contribute to the increase of survival and reverse of acute myocardial infarction remodeling 27. However, a sub-population of the STEMI patients managed by PCI with TIMI III revascularization might have a long-term negative impact of the STEMI on HF development due to late cardiac remodeling. In this context, the circulating level of sST2 measured at discharge may improve risk stratification scores based on clinical criteria, SYNTAX score, and other biomarker models 282930.
Previously, an elevated level of sST2 was reported as an independent predictor for survival in patients with several phenotypes of HF (HFrEF, HFmrEF, HFpEF), as well as for individuals with STEMI/non-STEMI after PCI and thrombolysis, stable coronary artery diseases, atrial fibrillation, diabetes mellitus 31323334. Although there is a wide spectrum of predictive biomarkers in STEMI patients undergoing PCI, sST2 appeared to be superior to NT-proBNP, cardiac troponins, myoglobin, CK-MB and clinical findings in prognostication of early STEMI complications and one-year major adverse cardiovascular and cerebrovascular events, defined as a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and ischemia-driven re-vascularization 3536. Recently, Jenkins et al. (2017) 16 reported that sST2 adversely impacted short-term prognosis after STEMI, which related to cardiac mechanical strain. Moreover, the authors studied a large patient cohort (n=1401) with incident myocardial infarction and found that half of STEMI patients enrolled in the investigation had an elevated level of sST2. Consequently, the elevated level of sST2 correlated with a large excess risk of death and HF independently of other prognostic biomarkers including comorbidities, Killip class, and troponin T. However, it has not been shown whether elevated levels of sST2 are best fitted to predict late cardiac remodeling after successful PCI.
In our study, the circulating level of sST2 at discharge (> 35 ng/mL) was the best predictor for late adverse cardiac remodeling, while other factors, such as age, T2DM, the mean value of SYNTAX score, NT-proBNP, the peak level of troponin I were insufficient to predict adverse cardiac remodeling. These findings were probably related to the design of the study. Indeed, we did not include patients with recurrent myocardial infarction and established HF, for whom conventional cardiovascular risk factors could be much more. Therefore, we scrutinized STEMI patients with completed re-vascularization (TIMI III), and the serum level of sST2 was measured independently before discharging from the hospital. As a result, we confirmed that serious coronary causes such as left main and multiple damaged coronary arteries were additional predictors of late adverse cardiac remodeling apart from the elevated level of sST2. Indeed, previous studies strongly supported the role of sST2 as a greater prognosticator for 30-day mortality and cardiac remodeling after discharge in STEMI patients compared to TnI 37. At the same time, the sST2 cut-off value of 35 ng/mL could distinguish patients that died within 30 days from those who had no cardiovascular events including death in both STEMI and non-STEMI patients 3038. We are the first group reported the correlation between the serum level of sST2 (> 35 ng/mL) and the late cardiac remodeling. The predictive power of sST2 seems to increase proportionally to the cut-off value of >35 ng/mL, which is a subject for further investigations.
A possible explanation for our findings is microvascular obstruction which frequently accompanies STEMI managed by PCI 39. Weir et al. (2010) 40 reported that the level of sST2 was significantly higher in STEMI patients with greater infarct transmurality and endocardial extent, and in the presence of microvascular obstructions. Moreover, authors showed that serum sST2 significantly correlated with LVEF at the baseline and 24 weeks after STEMI, changes in sST2 correlated with changes in LVEDV index. These data strongly support our results. Probably, microvascular inflammation due to obstruction of small-sized coronary arteries after PCI and severe subsequent atherosclerosis of large coronary arteries can trigger the sST2 release from cardiac myocytes and fibroblasts. Additionally, cardiac stretching due to post-myocardial infarction left ventricular dilation could be a possible factor associated with the elevation of sST2 in serum. A small proportion of T2DM patients and lack of individuals with severe declining eGFR in our study were not probably important factors for discharged elevation of sST2 after PCI. Thus, we could suggest that co-existing coronary reasons (severe left main, multiple coronary artery stenosis, and microvascular obstruction) are causative factors contributing to late cardiac remodeling in STEMI patients with PCI TIMI III re-vascularization.
Conclusions
We have shown that the circulating level of sST2 measured at discharge in acute STEMI patients managed by PCI could predict late adverse LV remodeling six months post-PCI. These findings provide a new approach to stratify patients with successful coronary re-vascularization at risk of HF.
Competing Interests
There are no conflicts of interest
Authors' Contributions
Conception and design: Olga V. Petyunina; writing of the article Olga V Petyunina, Mykola P. Kopytsya, Alexander E. Berezin; critical revision of the article for intellectual content Alexander E Berezin.
Acknowledgments
There are no previous presentations of the information reported in the article. We thank Nataliia Tytarenko and Igor Polivenok for performing ultrasound examination and cardiac interventions respectively. Additionally, we thank, Galina Bugrimenko for her excellent technical assistance. Permission to acknowledge has been obtained.
Founding
The study is a fragment of the research project: “To study the biochemical, genetic mechanisms of reperfusion damage of the myocardium and to assess the cardioprotective effect of antiplatelet therapy in acute myocardial infarction”, State Registration No. 0117U003028 / Ukraine.
References
-
Hassanin
A.,
Brener
S. J.,
Lansky
A. J.,
Xu
K.,
Stone
G. W..
Prognostic impact of multivessel versus culprit vessel only percutaneous intervention for patients with multivessel coronary artery disease presenting with acute coronary syndrome. EuroIntervention.
2015;
11
:
293-300
.
View Article PubMed Google Scholar -
Fukutomi
M.,
Toriumi
S.,
Ogoyama
Y.,
Oba
Y.,
Takahashi
M.,
Funayama
H..
Outcome of staged percutaneous coronary intervention within two weeks from admission in patients with ST-segment elevation myocardial infarction with multivessel disease. Catheterization and Cardiovascular Interventions.
2018;
23
:
e27896
.
View Article PubMed Google Scholar -
Kobayashi
Y.,
Lonborg
J.,
Jong
A.,
Nishi
T.,
Bruyne
B. De,
Hofsten
D. E.,
Danami-3-Primulti
Fame,
null
null.
Prognostic Value of the Residual SYNTAX Score After Functionally Complete Revascularization in ACS. Journal of the American College of Cardiology.
2018;
72
:
1321-9
.
View Article PubMed Google Scholar -
Genereux
P.,
Madhavan
M. V.,
Mintz
G. S.,
Maehara
A.,
Palmerini
T.,
Lasalle
L..
Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. Journal of the American College of Cardiology.
2014;
63
:
1845-54
.
View Article PubMed Google Scholar -
Ibanez
B.,
James
S.,
Agewall
S.,
Antunes
M. J.,
Bucciarelli-Ducci
C.,
Bueno
H.,
null
null.
2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal.
2018;
39
:
119-77
.
View Article PubMed Google Scholar -
Zhao
X. D.,
Zhao
G. Q.,
Wang
X.,
Shi
S. T.,
Zheng
W.,
Guo
R. F..
Optimal timing of staged percutaneous coronary intervention in ST-segment elevation myocardial infarction patients with multivessel disease. Journal of Geriatric Cardiology : JGC.
2018;
15
:
356-62
.
View Article PubMed Google Scholar -
Stone
G. W.,
Genereux
P.,
Harrington
R. A.,
White
H. D.,
Gibson
C. M.,
Steg
P. G..
Impact of lesion complexity on peri-procedural adverse events and the benefit of potent intravenous platelet adenosine diphosphate receptor inhibition after percutaneous coronary intervention: core laboratory analysis from 10 854 patients from the CHAMPION PHOENIX trial. European Heart Journal.
2018;
ooo
.
View Article PubMed Google Scholar -
Vanezis
A. P.,
Arnold
J. R.,
Rodrigo
G.,
Lai
F. Y.,
Debiec
R.,
Nazir
S..
Daily remote ischaemic conditioning following acute myocardial infarction: a randomised controlled trial. Heart (British Cardiac Society).
2018;
104
:
1955-62
.
View Article PubMed Google Scholar -
Lopes
R. D.,
Silva
P. G. de Barros E,
Jesuino
I. de Andrade,
Santucci
E. V.,
Barbosa
L. M.,
Damiani
L. P..
Timing of Loading Dose of Atorvastatin in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndromes: Insights From the SECURE-PCI Randomized Clinical Trial. JAMA Cardiology.
2018;
3
:
1113-8
.
View Article PubMed Google Scholar -
Wang
Z.,
Dai
H.,
Xing
M.,
Yu
Z.,
Lin
X.,
Wang
S..
Effect of a single high loading dose of rosuvastatin on percutaneous coronary intervention for acute coronary syndromes. Journal of Cardiovascular Pharmacology and Therapeutics.
2013;
18
:
327-33
.
View Article PubMed Google Scholar -
Kastrati
A.,
Mehilli
J.,
Neumann
F. J.,
Dotzer
F.,
Berg
J. ten,
Bollwein
H.,
Intracoronary
Stenting,
Trial
Investigators Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2.
Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. Journal of the American Medical Association.
2006;
295
:
1531-8
.
View Article PubMed Google Scholar -
Liu
T.,
Xie
Y.,
Zhou
Y. J.,
Li
Y. P.,
Ma
H. Y.,
Guo
Y. H..
Effects of upstream tirofiban versus downstream tirofiban on myocardial damage and 180-day clinical outcomes in high-risk acute coronary syndromes patients undergoing percutaneous coronary interventions. Chinese Medical Journal.
2009;
122
:
1732-7
.
PubMed Google Scholar -
Berezin
A. E..
Biomarkers in heart failure. Journal of Blood & Lymph.
2017;
7
:
172-9
.
-
Januzzi
J. L..
ST2 as a cardiovascular risk biomarker: from the bench to the bedside. Journal of Cardiovascular Translational Research.
2013;
6
:
493-500
.
View Article Google Scholar -
Hartopo
A. B.,
Sukmasari
I.,
Puspitawati
I..
The Utility of Point of Care Test for Soluble ST2 in Predicting Adverse Cardiac Events during Acute Care of ST-Segment Elevation Myocardial Infarction. Cardiology Research and Practice.
2018;
2018
:
3048941
.
View Article PubMed Google Scholar -
Jenkins
W. S.,
Roger
V. L.,
Jaffe
A. S.,
Weston
S. A.,
AbouEzzeddine
O. F.,
Jiang
R..
Prognostic Value of Soluble ST2 After Myocardial Infarction: A Community Perspective. The American Journal of Medicine.
2017;
130
:
1112.e9-15
.
View Article Google Scholar -
Austen
W. G.,
Edwards
J. E.,
Frye
R. L.,
Gensini
G. G.,
Gott
V. L.,
Griffith
L. S..
A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation.
1975;
51
:
5-40
.
View Article PubMed Google Scholar -
Morrow
D. A.,
Antman
E. M.,
Charlesworth
A.,
Cairns
R.,
Murphy
S. A.,
Lemos
J. A. de.
TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation.
2000;
102
:
2031-7
.
View Article PubMed Google Scholar -
Kappetein
A. P.,
Dawkins
K. D.,
Mohr
F. W.,
Morice
M. C.,
Mack
M. J.,
Russell
M. E..
Current percutaneous coronary intervention and coronary artery bypass grafting practices for three-vessel and left main coronary artery disease. Insights from the SYNTAX run-in phase. European Journal of Cardio-Thoracic Surgery.
2006;
29
:
486-91
.
View Article PubMed Google Scholar -
Catapano
A. L.,
Graham
I.,
Backer
G. De,
Wiklund
O.,
Chapman
M. J.,
Drexel
H.,
Force
Members Authors/Task.
2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis.
2016;
253
:
281-344
.
View Article PubMed Google Scholar -
Williams
B.,
Mancia
G.,
Spiering
W.,
Rosei
E. Agabiti,
Azizi
M.,
Burnier
M..
2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal.
2018;
39
:
3021-104
.
View Article Google Scholar -
Standards of Medical Care in Diabetes—2017: Summary of Revisions. Diabetes Care.
2017;
40
:
S4-S5
.
-
Galderisi
M.,
Henein
M. Y.,
D\'Hooge
J.,
Sicari
R.,
Badano
L. P.,
Zamorano
J. L.,
of
Echocardiography European Association.
Recommendations of the European Association of Echocardiography: how to use echo-Doppler in clinical trials: different modalities for different purposes. European Journal of Echocardiography.
2011;
12
:
339-53
.
View Article PubMed Google Scholar -
Lang
R. M.,
Bierig
M.,
Devereux
R. B.,
Flachskampf
F. A.,
Foster
E.,
Pellikka
P. A.,
Writing
Group Chamber Quantification,
Echocardiography\'s
Guidelines American Society of,
Standards
Committee,
of
Echocardiography European Association.
Recommendations for chamber quantification: a report from the American Society of Echocardiography\'s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography.
2005;
18
:
1440-63
.
View Article PubMed Google Scholar -
Lupon
J.,
Gaggin
H. K.,
Antonio
M. de,
Domingo
M.,
Galan
A.,
Zamora
E..
Biomarker-assist score for reverse remodeling prediction in heart failure: the ST2-R2 score. International Journal of Cardiology.
2015;
184
:
337-43
.
View Article PubMed Google Scholar -
Levey
A. S.,
Stevens
L. A.,
Schmid
C. H.,
Zhang
Y. L.,
Castro
A. F.,
Feldman
H. I.,
Ckd
E. P. I..
A new equation to estimate glomerular filtration rate. Annals of Internal Medicine.
2009;
150
:
604-12
.
View Article Google Scholar -
Pasceri
V.,
Patti
G.,
Pelliccia
F.,
Gaudio
C.,
Speciale
G.,
Mehran
R..
Complete Revascularization During Primary Percutaneous Coronary Intervention Reduces Death and Myocardial Infarction in Patients With Multivessel Disease: Meta-Analysis and Meta-Regression of Randomized Trials. JACC: Cardiovascular Interventions.
2018;
11
:
833-43
.
View Article PubMed Google Scholar -
Marino
R.,
Magrini
L.,
Orsini
F.,
Russo
V.,
Cardelli
P.,
Salerno
G.,
Great
Network.
Comparison Between Soluble ST2 and High-Sensitivity Troponin I in Predicting Short-Term Mortality for Patients Presenting to the Emergency Department With Chest Pain. Annals of Laboratory Medicine.
2017;
37
:
137-46
.
View Article Google Scholar -
Breidthardt
T.,
Balmelli
C.,
Twerenbold
R.,
Mosimann
T.,
Espinola
J.,
Haaf
P..
Heart failure therapy-induced early ST2 changes may offer long-term therapy guidance. Journal of Cardiac Failure.
2013;
19
:
821-8
.
View Article PubMed Google Scholar -
Kohli
P.,
Bonaca
M. P.,
Kakkar
R.,
Kudinova
A. Y.,
Scirica
B. M.,
Sabatine
M. S..
Role of ST2 in non-ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 trial. Clinical Chemistry.
2012;
58
:
257-66
.
View Article Google Scholar -
Manzano-Fernandez
S.,
Januzzi
J. L.,
Pastor-Perez
F. J.,
Bonaque-Gonzalez
J. C.,
Boronat-Garcia
M.,
Pascual-Figal
D. A..
Serial monitoring of soluble interleukin family member ST2 in patients with acutely decompensated heart failure. Cardiology.
2012;
122
:
158-66
.
View Article PubMed Google Scholar -
Huang
W. P.,
Zheng
X.,
He
L.,
Su
X.,
Liu
C. W.,
Wu
M. X..
Role of Soluble ST2 Levels and Beta-Blockers Dosage on Cardiovascular Events of Patients with Unselected ST-Segment Elevation Myocardial Infarction. Chinese Medical Journal.
2018;
131
:
1282-8
.
View Article PubMed Google Scholar -
Miller
A. M.,
Purves
D.,
McConnachie
A.,
Asquith
D. L.,
Batty
G. D.,
Burns
H..
Soluble ST2 associates with diabetes but not established cardiovascular risk factors: a new inflammatory pathway of relevance to diabetes?. PLoS One.
2012;
7
:
e47830
.
View Article Google Scholar -
Farcaş
Anca Daniela,
Anton
Florin Petru,
Goidescu
Cerasela Mihaela,
Gavrilă
Iulia Laura,
Vida-Simiti
Luminiţa Animarie,
Stoia
Mirela Anca.
Serum Soluble ST2 and Diastolic Dysfunction in Hypertensive Patients. Disease markers.
2017;
2017
.
-
Yu
J.,
Oh
P. C.,
Kim
M.,
Moon
J.,
Park
Y. M.,
Lee
K..
Improved early risk stratification of patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention using a combination of serum soluble ST2 and NT-proBNP. PLoS One.
2017;
12
:
e0182829
.
View Article Google Scholar -
Schernthaner
C.,
Lichtenauer
M.,
Wernly
B.,
Paar
V.,
Pistulli
R.,
Rohm
I..
Multibiomarker analysis in patients with acute myocardial infarction. European Journal of Clinical Investigation.
2017;
47
:
638-48
.
View Article PubMed Google Scholar -
Marino
R.,
Magrini
L.,
Orsini
F.,
Russo
V.,
Cardelli
P.,
Salerno
G.,
Great
Network.
Comparison Between Soluble ST2 and High-Sensitivity Troponin I in Predicting Short-Term Mortality for Patients Presenting to the Emergency Department With Chest Pain. Annals of Laboratory Medicine.
2017;
37
:
137-46
.
View Article PubMed Google Scholar -
Sabatine
M. S.,
Morrow
D. A.,
Higgins
L. J.,
MacGillivray
C.,
Guo
W.,
Bode
C..
Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation.
2008;
117
:
1936-44
.
View Article PubMed Google Scholar -
Berezin
A. E.,
Samura
T. A..
Prognostic value of biological markers in myocardial infarction patients. Asian Cardiovascular & Thoracic Annals.
2013;
21
:
142-50
.
View Article PubMed Google Scholar -
Weir
R. A.,
Miller
A. M.,
Murphy
G. E.,
Clements
S.,
Steedman
T.,
Connell
J. M..
Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. Journal of the American College of Cardiology.
2010;
55
:
243-50
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 12 (2018)
Page No.: 2863-2875
Published on: 2018-12-08
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6528 times
- Download PDF downloaded - 2038 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress





