Abstract
Background: Oxidative stress is the main cause of mortality in chemical burn-induced esophageal injury. Melanin, a natural antioxidant compound from yeast-like fungus Nadsoniella nigra strain X- 1, has been shown to decrease the content of lipid peroxidation products after burn. The aim of this study was to investigate the protective effect of melanin in the treatment of esophageal injury after a chemical burn.
Methods: A alkali burn model was used to induce injury to the esophagus in immature rats. Changes in the levels of malondialdehyde, secondary products of lipid peroxidation (thiobarbituric acid reactive substances), superoxidase dismutase, and catalase in the blood, as well as changes in the esophagus tissue, were examined.
Results: Melanin decreased the content of lipid peroxidation products following burn injury. Melanin increased the activity of superoxidase dismutase and reduced the activity of catalase, as well as reduced esophagus oxidative injury in our chemical burn model.
Conclusion: Melanin treatment may protect against chemical burninduced esophageal injury, possibly by inhibiting burn-induced oxidative stress.
Introduction
Esophageal alkaline chemical burns appear to be a serious health problem that lack effective treatment options 1. The majority of these events occur due to accidental swallowing of alkaline chemicals because of their wide household use. The basic histo-pathologic reaction of a tissue subjected to alkaline burn is collagen synthesis, deposition, and remodeling. In the case of esophageal wall full thickness injuries, the normal esophageal tissues are replaced by dense connective one 2.
According to statistics, the rate of these burns in Ukraine is 21.3 per 10 000 of the population, which corresponds to more than 30% of all burns received by children under the age of 5 years. In addition, such burns occupy the third place among all types of childhood injuries. Esophagus chemical burn treatment represents one of the most complex specific problems of pediatric medicine, despite all the achievements in this field. Tissue structure immaturity in children, as well as imperfections of vital organ function, are the main cause of prolonged whole organism pathological disorders 3.
The grave degree of different organ pathological reactions, and varying disease outcomes, are usually dependent on burn injury severity, age of child, and proper therapy selection 4. Moreover, excessive lipid peroxidation activation is also a factor 5. As a result of the increased activity of free radical processes and a weakening antioxidant defense (AOD) system, toxic products can accumulate, causing severe metabolic disturbances with general oxidative stress formation 6.
It is already known that activation of lipid peroxide (LP) oxidation (LPO) causes cell membrane oxidative damage, enzyme catalytic activity inhibition, as well as other harmful effects which compromise various vital cell functions 7. Under the conditions of enhanced LPO processes, the cell’s antioxidant system play an important role because their enzymatic components are involved in the regulation of free radical formation as well as degradation of LP product 8910. Burn conditions violate the homeostatic balance between active oxygen synthesis and antioxidant system functions 5. The antioxidant activity of phenolic compounds occurs through various action mechanisms: inhibition of the active form of oxygen formation, singlet oxygen molecule neutralization, metal ion binding (as the reaction catalysts for the active oxygen forms), and interruption of the free radical reaction cascade during the LP processes 1112.
Analysis of modern literature has suggested possible promising means of lipid peroxidation normalization during first and second degree esophageal burns, in the case of natural origin substances, based on polyphenolic structure. These substances include melanin, a product of the yeast-like fungus Nadsoniella nigra strain X-1 13. It is known that this drug exhibits antioxidant 141516, immunomodulatory 1718, anti-carcinogenic 19 and stress-relieving 20 properties, allowing it to be widely used in medicine.
Therefore, the purpose of this work was to evaluate the peroxidation product content and activity changes in antioxidant system enzymes, e.g. superoxide dismutase (SOD) and catalase, in rat blood serum and tissues throughout different stages after an experimental burn infliction, with or without melanin treatment following burn injury.
Methods
Burn model of animals
I our experiments, white wild rats (1 month old, 90-110 g in weight) were used in full compliance with provisions for the use of animals in biomedical experiments, as approved by the First Ukrainian National Congress on Bioethics (September 2001), as well as other international agreements and national legislation in the area. Chemical burns in animals were experimentally inflicted in the following way: alkaline esophageal burn (АEB) was induced using 20% sodium hydroxide (NaOH). For this, the probe was injected into both an esophagus soldered at the end and a hole at a distance of 2 mm from it. The probe of 0.2 mL solution of 20% NaOH was slowly injected for a depth of 4 cm from the rat’s upper incision. This burn model corresponded to a 2nd degree burn injury. Control rats were administered with the respective amount of water, injected orally once 21. All animals in the study received a standard diet.
Experimental groups
The experimental scheme was as follows:
Group 1 — control, healthy rats (intact control);
Group 2 — rats bearing 2nd degree AЕВ, administered with appropriate dose and timing of saline (burn control);
Group 3 — rats bearing 2nd degree AЕВ, administered with melanin starting from the 2nd day of the experiment at a dose of 1 mg/kg for 14 days.
Treatment with melanin
Producers of melanin used in our studies were the yeast like fungi Nadsoniella nigra strain X1 taken from the vertical rock samples from Antarctica island Galindez 20.
Sample collection
Samples from the rats were collected at the 7th, 15th and 21st day of the experiment, according to the stage of burn disease 22. The method of sacrificing animals was cervical dislocation. The parameters that were evaluated were measured from serum, which was isolated by centrifugation of blood at 2000 g × 40 min, and from esophageal tissue.
Determin ation of oxidative stress levels and antioxidant enzyme activities
The TBK-active product content of was determined by Haryshvyly & Stalnaya (1977) 23, while diene conjugate (DK) amount — by Gavrilov (1988) 24. The enzyme activities were determined as follows: superoxide dismutase (SOD.KF 1.15.1.1) by the method described previously 25; catalase (CAT, KF 1.11.1.9) by the method described previously 26. The tissue protein content was estimated by the Lowry method 27.
Statistical analysis
The experimental data were statistically analysed using Student's t-test; the significance level was set as p <0.05.
Results
The body’s antioxidant protection system controls and inhibits all phases of free radical reactions, from their initiation to the formation of hydroperoxides and TBK-active products. Therefore, in the first stage of our study, we determined the LPO intensity, measuring the level of TBK-active products and malondialdehyde (MDA) in rat blood serum and esophagus homogenate samples.
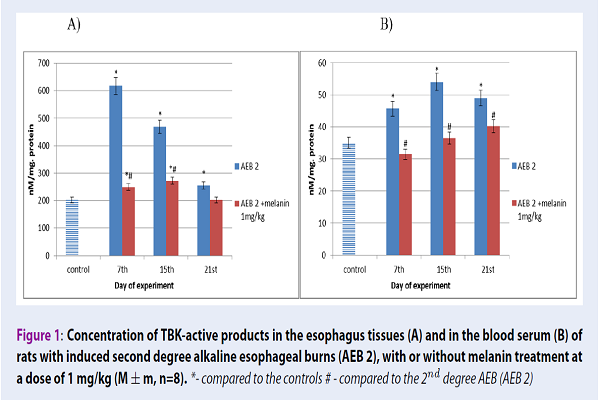
In our 2nd degree AEB model, throughout the study, the concentration of TBK-active products in rat blood serum significantly increased by 30%, 54% and 40% after 7, 15 and 21 days, respectively, in comparison with the control values. Similar results were obtained in the esophageal tissues, where the TBK-active product concentration after 2nd degree AEB increased by 204%, 131% and 25% after 7, 15 and 21 days of our experiment, respectively, if compared to the control. When the drug melanin was administered, there was a tendency of the TBK-active product amount to decrease in rat blood serum by 31% at the 7th day, by 32% at the 15th day, and by 18% at the 21st day. In the esophagus tissues, this decrease was decreased by 61% at the 7th day, by 42% at the 15th day and by 20% at the 21st day of the experiments, respectively, when compared with animals bearing 2nd degree AEB (Figure 1).
A similar difference was also found in the esophagus tissues and rat blood serum when studying the process of lipid peroxidation and formation of the main end product, malondialdehyde (MDA), which is formed during primary and secondary lipid peroxidation product degradation. Indeed, for 2nd degree AEB animals, the concentration of MDA in rat blood serum throughout the experiment was significantly increased by 246%, 387% and 556% at the 7th, 15th and 21st day of experiment, respectively, in comparison with the controls. The results obtained in the esophagus tissues were somewhat similar, as the MDA concentration for AEB 2 increased by 357%, 473% and 589% at the 7th, 15th and 21st day of the experiment, respectively, when compared to the control. When the drug melanin was administered, it provoked the MDA content to decrease in both rat blood serum and esophagus tissue, especially after 15 and 21 days. In the serum, the MDA concentration was reduced by 43% and 62% at the 15th and 21st day, when compared to the values for 2nd degree AEB. In the esophagus tissues, the MDA amount was diminished by 57% at the 15th day and by 82% at the 21st day, in comparison to the 2nd degree AEB values (Figure 2).
From analysis of the SOD activity in rat blood serum from animals with 2nd degree AEB, it was observed that the enzyme activity of SOD decreased by 40% at the 7th day, by 47% at the 15th day and by 20% at the 21st day of the experiment, in comparison with the control. In the study of the esophagus tissues, an increase in SOD activity by 11% was observed at the 7th day, which may be attributed to the body’s compensatory response to burns, while at the 15th day and 21st day, SOD activity decreased by 33% and 50%, respectively, when compared to the control values. When the drug melanin was administered, there was an observed increase in the blood serum SOD activity by 33% at the 7th day, by 63% at the 15th day, and by 17% after the 21st day, in comparison to the data obtained from rats with 2nd degree AEB. In the esophageal tissues, under the action of melanin, this activity was increased by 33% at the 21st day, when compared to the data obtained from the 2nd degree AEB animals (Figure 3).
We showed that under the conditions of 2nd degree AEB, the catalase activity in rat blood serum increased throughout the experiment - by 106% at the 7th day, by 153% at the 15th day, and by 130% at the 21st day, when compared to the control values. In the esophagus tissues, the activity of this enzyme was higher by 502%, 373% and 136% after 7, 15 and 21 days, respectively, in comparison with the controls. Since hydrogen peroxide easily penetrates plasma membranes, catalase activation is more likely to increase in response to normal hydrogen peroxide levels in the esophageal tissues. With the introduction of the drug melanin, the catalase activity decreased in the esophagus by 52% at the 7th day, by 37% at the 15th day and by 19% at the 21st day, when compared to the values from animals with 2nd degree AEB. The growth of catalase activity may indicate that this enzyme has an active organism LPO resistance in the organism in the processes after chemical burn infliction (Figure 4).
Therefore, with 2nd degree esophagus burns on the background of increased LOP activity, we demonstrated the significant inhibition of antioxidant defense mechanisms. Under the conditions of burn disease, a disturbance of the balance between the pro-oxidant factor activity and the antioxidant system activity may be present due to the intensification of the free radical conversion process. It was established that the evaluated system activity changes, in the case of AEB, depend on the stage of burn disease. The highest amount of TBK-active products was observed at toxemia (7 day) and septicotoxemia (15 day) stages. The greatest increase in the MDA level was evident in the blood and esophagus tissues at the 15th and 21st days. There was also a decrease in SOD activity and increase in catalase activity. Finally, it was shown that the use of melanin significantly contributes to the reduction of peroxide oxidation product content (MDA, TBK-active products), as well as contributing to SOD activity increase and catalase activity decrease.
Discussion
In this study, the antioxidant property of melanin was studied in an alkaline esophageal burn model. Our results suggest that melanin is an inhibitor of lipid peroxide oxidation.
Active forms of oxygen (AFO) hyper-production, being one of the main pathological factors, induces the literal “avalanche” of uncontrolled AFО synthesis — the process of a huge potential danger for any cell and tissue. One of the decisive factors in damaged epithelium regeneration is the oxidant-antioxidant balance restoration speed. This process can be aided effectively using various antioxidants. The radicals of hydroxyl and superoxide anion are two types of the most important free radicals in whole organisms. The production of hydroxyl radicals plays a significant role in the initiation of lipid peroxidation 28. Membrane lipids are particularly susceptible to oxidation due to the high concentration of polyunsaturated fatty acids and their association with enzymatic and non-enzymatic systems in the cell membrane is capable of generating free molecules of radicals 29.
The oxidative modification of cellular structures and enzymes appears to be one of their destruction mechanisms leading to the subsequent molecular component upgrade. The process of radical oxidation is associated with protein lipid, nucleic acids, prostaglandin and other substance turnover in cells. Antioxidant system exhaustion usually become a major factor of pathogenesis for a considerable number of diseases.
Normally free radical processes play an important role in biological system functioning by participating in complex reaction sets aimed for regulation of cellular metabolism. In addition, free radical reactions are considered to be the universal mechanism of cell damage inflicted by various factors 30. Thus, their excessive activation plays a key role in cell damage, being able to stimulate both cell defense mechanisms and proliferation. The excessive accumulation of LP products in an organism occurs mainly in a form of highly toxic superoxide anion-radicals, which can lead to significant organism disorders and severe endotoxicosis.
Tissue and serum content of MDA and TBK-active products (the final product of lipid breakdown caused by oxidative stress) are considered to be good indicators for radical-induced lipid peroxidation3132. After burning, the content of lipid peroxide oxidation increased. In the application of melanin, there was a decrease in the content of the studied parameters, which may indicate the inhibition of lipid peroxidation by melanin.
Burn disease intensifies active oxygen form synthesis which can lead to prominent tissue damage. In this case, an unbalance between the intensity of the free radical formation process and the antioxidant system activity levels may be issued, resulting in bio-molecule oxidation speed increases. Disturbances in the normal course of oxidative processes underlying cell metabolism and determining organism overall adaptive capacity leads to the oxidative stress formation 33. It is a major metabolic syndrome that promotes the development of numerous organism morphofunctional disorders 34.
An excessive amount of ROS can react with many bio-molecules, such as DNA 35, lipids 36, or proteins 37. Our body has several enzymes that play an important role in removing excess ROS in the cell. Enzymes such as SOD and CAT are most important in the cellular antioxidant system 3839.
Superoxide dismutase (SOD) and catalase are the major enzymes of antioxidant cell defense. The activity of these enzymes determines cell resistance to oxidative stress consequences. Changes in the activity of SOD and catalase appear to be an indicator of formed primary product amounts in reactions of superoxide ion-radical oxidation as well as a number of its further transformation products. Therefore, in order to evaluate free radical processes activity in the tissues of both the esophagus and the rat blood serum, we studied the activity of the following enzyme components of the antioxidant system - catalase and SOD.
Superoxide dismutase is an enzyme belonging to a group of antioxidant enzymes protecting an organism from highly toxic oxygen radicals. SOD catalyzes superoxide dismutation into oxygen and hydrogen peroxide and is present in all cells able to absorb oxygen. Superoxide dismutase activity suppression usually leads to superoxide anion-radical accumulation, initiating chains of free radical reactions in cells. Therefore, the established SOD activity dynamics, as well as the LP final product concentration, may indicate free radical oxidation process activation 40.
In systemic metabolic disorder development inflicted by burns of mild and moderate severity, free radical lipid oxidation plays an important role in the case of both enzymatic and non-enzymatic antioxidant system activity and their insufficient levels in blood 29. The increase of free radical synthesis activity in such pathological conditions leads to prooxidant-antioxidant balance disturbance and, as an effect, oxidative stress development.
Catalase (hydrogen peroxide: hydrogen peroxide oxidoreductase, 1.11.1.6.) is an enzyme of the oxidoreductase class found in almost all eukaryotic organism tissues. It carries out the reaction of reducing hydrogen peroxide to water and oxygen using various proton donors. SOD and catalase act as synergistic enzymes. Thus, in the process of the SOD reaction, hydrogen peroxide is formed, for which the destruction of a catalase is required. If catalase activity is diminished, hydrogen peroxide can act as a SOD inhibitor 41. Therefore, correct and sufficient mutual activities of these two enzymes are very important for maintaining the cell oxidant-antioxidant balance.
As it is known, the increased number of active forms of oxygen can be caused by the activation of neutrophils, which in turn can be activated by interleukins. Peroxisol proliferation activation receptors (PPARs) are considered one of such molecular links between pro-inflammatory cytokines and transcription factors. The earlier studies indicate that the effect of melanin may be mediated by interaction or partial interaction with receptors 42.
Therefore, it was interesting to investigate the activity of these enzymes under conditions of chemical burn and also under conditions of application of melanina as treatment. At the burn, the activity of SOD is significantly decreased and catalase activity is increased in blood and esophageal tissues. elanin increased the enzymatic activity of SOD and decreased the activity of catalase (Figure 3Figure 4). This may be responsible for increased resistance to oxidative stress. As shown in Figure 4C, the activity of catalase in the blood serum and tissues of the esophagus was significantly increased after burns, these data may be due to an instinctive protective effect in response of oxidative stress.
Conclusions
To our knowledge, this is the first study to demonstrate the antioxidant property of melanin in an alkaline burn model of the esophagus. The study herein showed that melanin reduced the content of lipid peroxide oxidation products and normalized antioxidant enzyme activity after induction of alkaline esophageal burn. The data obtained in the study indicate that there is a possible prospect for the use of melanin in the future for treating chemical burns of the esophagus.
Competing Interests
No conflict of interest to declare.
Authors' Contributions
Natalia Chornenka: planning an experiment, carrying out of experimental researches, calculation of results.
Yana Raetska: planning an experiment, analysis of results, carrying out of experimental researches.
Dmitro Grebinyk: preparation of the article for printing, calculation of results.
Alevtina Dranitsina: carrying out of experimental researches, calculation of results.
Olexiy Savchuk: planning an experiment, analysis of results, carrying out of experimental researches.
Tetiana Beregova: preparation of the article for printing.
Ludmila Ostapchenko: planning an experiment, analysis of results
Acknowledgments
We would like to appreciation Professor Tatiana Beregova, Ph.D., for kindly providing the melanin reagent for our research.
Abbreviations
AEB: alkaline esophageal burn
AFO: active forms of oxygen
AOD: antioxidant defense
CAT: catalase
DK: diene conjugate
LP: lipid peroxide
LPO: lipid peroxide oxidation
MDA: malondialdehyde
PPARs: peroxisol proliferation activation receptors
SOD: superoxide dismutase
References
-
Chipp
E.,
Charles
L.,
Thomas
C.,
Whiting
K.,
Moiemen
N.,
Wilson
Y..
A prospective study of time to healing and hypertrophic scarring in paediatric burns: every day counts. Burns and Trauma.
2017;
5
:
3
.
PubMed Google Scholar -
Chipp
E.,
Milner
C. S.,
Blackburn
A. V..
Sepsis in burns: a review of current practice and future therapies. Annals of Plastic Surgery.
2010;
65
:
228-36
.
PubMed Google Scholar -
Alnababtah
K.,
Khan
S.,
Ashford
R..
Socio-demographic factors and the prevalence of burns in children: an overview of the literature. Paediatrics and International Child Health.
2016;
36
:
45-51
.
PubMed Google Scholar -
Jun
W. Hai,
Jie
X.,
Jun
Z.,
Feng
T.,
Hui
H. G..
Comparable results of epidemiology of children with burns among different decades in a burn unit in JinZhou, China. Burns.
2011;
37
:
513-20
.
PubMed Google Scholar -
Netyukhailo
L. G.,
Sukhomlin
T. A.,
Basarab
Y. A.,
Bondarenko
V. V.,
Kharchenko
S. V..
The state of the antioxidant system of the internal organs in rats during burn disease. Bûlleten\'Sibirskoj Mediciny.
2014;
13
:
51-5
.
-
Parihar
A.,
Parihar
M. S.,
Milner
S.,
Bhat
S..
Oxidative stress and anti-oxidative mobilization in burn injury. Burns.
2008;
34
:
6-17
.
PubMed Google Scholar -
L.G.
Net'ukhaylo,
v
Reactive oxygen. Young Scientist.
2014;
9
(12)
.
-
Net’ukhaylo LG
Kharchenko SV, Kostenko АG.
Pathogenesis of the burn disease. Svit medicini ta biologiyi.
2011;
1
.
-
Lavryshyn YY
Varkholyak IS, Martyschuk TV, Guta ZA, Ivankiv LB, Paladischuk OR, Murska SD, Gutyj BV, Gufriy DF..
The biological significance of the antioxidant defense system of animals body. Scientific Messenger LNUVMBT named after SZ Gzhytskyj..
2016;
18
:
66
.
-
NV
Kuz\'mina,
Sachko RG,
MM
Akymyshyn.
Aktyvnist\' enzymiv antyoksydantnogo zahystu v reproduktyvnyh organah koriv za normy ta patologii. Nauk. visnyk LNUVMBT im. S.Z. G\'zhyc\'kogo. L\'viv..
2014;
2
:
3-9
.
-
Carletti
G.,
Nervo
G.,
Cattivelli
L..
Flavonoids and Melanins: a common strategy across two kingdoms. International Journal of Biological Sciences.
2014;
10
:
1159-70
.
PubMed Google Scholar -
Pourcel
L.,
Routaboul
J. M.,
Cheynier
V.,
Lepiniec
L.,
Debeaujon
I..
Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends in Plant Science.
2007;
12
:
29-36
.
-
Chyzhanska NV
Tsyryuk OI, Beregova TV.
The level of cortisol in the blood of rats before and after stress action against the background of melanin. Visnik of problems of biology and medicine.
2007;
:
40-4
.
-
Brenner
M.,
Hearing
V. J..
The protective role of melanin against UV damage in human skin. Photochemistry and Photobiology.
2008;
84
:
539-49
.
-
Romanovskaia VA
Tashirev AB, Shilin SO, Chernaia NA..
Resistance to UV radiation of microorganisms isolated from the rock biotopes of the Antarctic region. Mikrobiolohichnyi zhurnal (Kiev, Ukraine: 1993).
2010;
72
:
8-13
.
-
Keypour
S.,
Riahi
H.,
Moradali
M.,
Rafati
H..
Investigation of the antibacterial activity of a chloroform extract of Ling Zhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae), from Iran. International Journal of Medicinal Mushrooms.
2008;
10
:
345-9
.
-
Racca
S.,
Spaccamiglio
A.,
Esculapio
P.,
Abbadessa
G.,
Cangemi
L.,
DiCarlo
F..
Effects of swim stress and alpha-MSH acute pre-treatment on brain 5-HT transporter and corticosterone receptor. Pharmacology, Biochemistry, and Behavior.
2005;
81
:
894-900
.
-
Chornenka
N. M.,
Raetska
Y. A.,
Savchuk
O. M.,
Torgalo
E. O.,
Beregova
T. V.,
Ostapchenko
L. I..
Correction Parameters of Endogenous Intoxication in Experimental Burn Disease at the Stage of Toxemia. Research Journal Of Pharmaceutical Biological And Chemical Sciences.
2016;
7
:
1042
.
-
Seniuk
O.,
Gorovoj
L.,
Kovalev
V..
Anticancerogenic propertis of melaninglucan complex from higher fungi. 2009;
2009
:
142-9
.
-
Golyshkin DV
Falalyeyeva TM, Chyzhanska NV, Beregova TV, Ostapchenko LI.
White blood count of rats under stress-induced stomach lesions and the prophylactic administration of melanin. Ukrainian Antarctic Journal.
2015;
:
114
.
-
Raetska
Y. B.,
Ishchuk
T. V.,
Savchuk
O. M.,
Ostapchenko
L. I..
Experimental modeling of first-degree chemically-induced esophageal burns in rats. Medicinal Chemistry (Shariqah, United Arab Emirates).
2013;
15
:
30-4
.
-
Fistal
E. Y.,
Kozinets
G. P.,
al
G.E Samoilenko and.
CombustiologyKharkov 2004.
Google Scholar -
T.G. Haryshvyly,
Y.D. Stalnaya.
1977.
Google Scholar -
Gavrilov
V. B..
Izmerenie dienovykh conyugatov v plasme krovi po UF poglosheniyu geptanovykh i izopropanolnykh ekstractov / V.B. Gavrilov, A.R. Gavrilov, N.F. Khmara. Laboratornoe Delo.
1988;
2
:
60-3
.
-
Kostyuk VA,
AI
Potapovich,
ZhI
Kovaleva.
prostoy i chuvstvitelnyy metod opredeleniya SOD, osnovannyy na reaktsiy okisleniya kvertsetina. Voprosy medetsinskoy khimiy.
1990;
2
:
88-91
.
-
M.A.
Koroliuk.
Laboratory work 1988.
Google Scholar -
Lowry
Oliver H,
Rosebrough
Nira J,
Farr
A Lewis,
Randall
Rose J.
Protein measurement with the Folin phenol reagent. Journal of biological chemistry.
1951;
193
(1)
:
265-275
.
-
Yang
X.,
Bai
H.,
Cai
W.,
Li
J.,
Zhou
Q.,
Wang
Y..
Lycium barbarum polysaccharides reduce intestinal ischemia/reperfusion injuries in rats. Chemico-Biological Interactions.
2013;
204
:
166-72
.
PubMed Google Scholar -
Aruoma
O. I..
Free radicals, oxidative stress, and antioxidants in human health and disease. Journal of the American Oil Chemists\' Society.
1998;
75
:
199-212
.
-
N.P. Chesnokova.
Aktivaciya svobodnoradikalnogo okisleniya - efferentnoe zveno tipovyh patologicheskih processov - Saratov. Izd-vo SMU.
2006;
:
177
.
-
Wang
X.,
Hai
C. X.,
Liang
X.,
Yu
S. X.,
Zhang
W.,
Li
Y. L..
The protective effects of Acanthopanax senticosus Harms aqueous extracts against oxidative stress: role of Nrf2 and antioxidant enzymes. Journal of Ethnopharmacology.
2010;
127
:
424-32
.
PubMed Google Scholar -
Sato
Y.,
Itagaki
S.,
Oikawa
S.,
Ogura
J.,
Kobayashi
M.,
Hirano
T..
Protective effect of soy isoflavone genistein on ischemia-reperfusion in the rat small intestine. Biological & Pharmaceutical Bulletin.
2011;
34
:
1448-54
.
-
Mihalchik EV
Piterskaya YuA, Lipatova VA.
Aktivnost antioksidantnyh fermentov v rane pri glubokih ozhogah. Byulleten eksperimentalnoj biologii i mediciny.
2009;
147
:
696-699
.
-
Agaeva RK
Fastova IA.
Svobodnoradikalnoe okislenie v tkanyah tonkoj kishki, legkih i pecheni pri ozhogovom shoke. Medicinskij vestnik Bashkortostana.
2009;
4
:
113-115
.
-
Marnett
L. J..
Oxyradicals and DNA damage. Carcinogenesis.
2000;
21
:
361-70
.
-
Ylä-Herttuala
S..
Oxidized LDL and atherogenesis. Annals of the New York Academy of Sciences.
1999;
874
:
134-7
.
-
Stadtman
E. R.,
Levine
R. L..
Protein oxidation. Annals of the New York Academy of Sciences.
2000;
899
:
191-208
.
-
Wang
X.,
Ye
X. L.,
Liu
R.,
Chen
H. L.,
Bai
H.,
Liang
X..
Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chemico-Biological Interactions.
2010;
184
:
328-37
.
PubMed Google Scholar -
Yang
X.,
Bai
H.,
Cai
W.,
Li
J.,
Zhou
Q.,
Wang
Y..
Lycium barbarum polysaccharides reduce intestinal ischemia/reperfusion injuries in rats. Chemico-Biological Interactions.
2013;
204
:
166-72
.
-
Dosvyadchynska MR
Antonyak HL.
Lipid peroxidation and antioxidant system enzyme activity in rat lung under long term administration of aflatoxin b1. Біологія тварин.
2012;
14
:
1-2
.
-
Abdel-Wahhab
M. A.,
Abdel-Galil
M. M.,
El-Lithey
M..
Melatonin counteracts oxidative stress in rats fed an ochratoxin A contaminated diet. Journal of Pineal Research.
2005;
38
:
130-5
.
-
Kukharskyy VM
Kukharskyy M, Chyizhanska NV, Tsyryuk OI.
The involvement of peroxisome proliferator-activated receptors gamma in antiulcer action of melanin. Ukrainian antarctic jornal.
2009;
8
:
374-376
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 10 (2018)
Page No.: 2712-2718
Published on: 2018-10-04
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7659 times
- Download PDF downloaded - 2160 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress





