Bacterial Meningitis: a five-year retrospective study among patients who had attended at University of Gondar Teaching Hospital, Northwest Ethiopia
Abstract
Acute Bacterial Meningitis (ABM) is an important cause of death and long-term neurological disability. Recent Information on the relative frequency of the isolation and antibiotic susceptibility patterns of these pathogens is scarce in Ethiopia. This study was to document the microbial characteristics, the antibacterial sensitivity pattern, and seasonal variation of community acquired acute bacterial meningitis. The study was retrospective, conducted at university of Gondar referral hospital, serving the rural population of the northwest parts of Ethiopia. A total of three thousand and eighty five cerebrospinal fluid specimens submitted to the bacteriology laboratory for culture and antibiotic susceptibility patterns in a period between January 2006 and December 2010. Analysis of extracted data was performed using SPSS statistical software (Version 17). The etiological agent had been identified in 120 (3.8%) of the total 3,085 CSF samples by culture. Thirty-nine (32.5%) of them were infants below the age of 12 months. S. pneumoniae was the predominant pathogen accounting for 52 (43.3%) of the cases. Whereas N. meningitidis and H. influenzae accounted for 27(22.5%), and 12(10%), respectively. Other gram negative bacilli and S. aureus were isolated from 21(17.2%), and 11(9.2%) cases, respectively. Among gram positive organismsS.pneumoniae showed a high level of drug resistance against co-trimoxazole 44(84.3%). Among gram negative bacteria, N.meningitidis was found to be resistant to co-trimoxazole in 25(92.5%). E. coli and salmonella spp. were found to be resistant to most antibiotics except ciprofloxacin. Multiple drug resistance was observed in 58.3% of the isolates. S. pneumoniae remains the major etiological agent of Community Acquired Acute Bacterial Meningitis (CAABM) both in adults and children in the study area. 5.7% of S. pneumoniaewere resistances to penicillin. Further research should focus on preventable aspects CAABM of, especially pneumococcal vaccines, to reduce the disease burden.
Introduction
In the pre‐antibiotic era, bacterial meningitis was a fatal disease. Now a day, the incidence of bacterial meningitis is between 3 and 5 per 100,000 people per year and nearly half of them die even after complete medical attention (Meningococcal disease): the disease is even more common in developing countries. Many clinical and etiologic studies performed over the past 30 years have demonstrated that Haemophilus influenzae type b (Hib), Neisseria meningitidis and Streptococcus pneumoniae were the most common causative organisms of bacterial meningitis worldwide. But the proportion due to each organism varies among geographic regions Nur et al., 2008: The development and implementation of a conjugate vaccine against Hib in the 2007 have contributed directly to changes in the epidemiological profile of meningitis caused by Hib.
More recently, pneumococcal heptavalent conjugate vaccines were licensed and are expected to influence the epidemiology of the disease. Therefore, a review of the epidemiology of bacterial meningitis is important in order to make rational decisions concerning future prevention and control strategies.
Bacterial meningitis has for a long time been treated with a combination of penicillin/ampicillin and chloramphenicol, and this combination is still the widely recommended first choice in most of Africa. However the increasing frequency of reports of bacterial resistance in‐vitro to these drugs has raised concern that this choice may no longer be appropriate Kiwanuka and Mwanga, 2001.
Moreover, the recommended antibiotic treatment of bacterial meningitis has come under scrutiny following frequent reports of in‐vitro resistance by the common causative organisms to penicillin and chloramphenicol from EthiopiaCommey et al., 1994 and Uganda Kiwanuka and Mwanga, 2001. In addition to this there was no a recent study on prevalence of bacterial meningitidis on this region. There for conducting researches on the case so as to solve the problem was a crucial issue in the current health system.
Material and methods
This Laboratory‐based retrospective analysis of 3085 CSF cultures and sensitivity tests was conducted in Gondar University Referral Hospital Laboratory within a five‐year period between January 2006 and December 2010. The samples were collected from different wards of the hospital in sterile containers by physicians and delivered to the bacteriology laboratory within half an hour collection and samples were processed following the standard microbiological procedures by inoculating on blood agar, chocolate agar, and MacConkey agar plates [(Oxoid Ltd, Basingstoke, Hampshire, UK) prepared as per the manufacturer instruction] and incubated at 35‐ 37°C aerobically. The chocolate agar plates were incubated by putting them in a candle jar, which provided 5‐10% CO2 concentration to create a microareophilic condition for fastidious bacteria. After 20‐24 hours of incubation, the plates were examined for the presence of bacterial colonies. Plates, which did not show any growth, were further incubated for an additional 24 hours. Organisms were identified by standard microbiological methods, which included colony morphology, as well as staining, biochemical and serological testsCheesbrough, 2006 JawetzWHO, 1991. Antibiotic sensitivity test was conducted on pure culture isolates using the disc diffusion methodBauer et al.,1966for the commonly used antibiotics: ampicillin (10Fg), penicillin G (10IU), gentamicin (10Fg), chloramphenicol (30Fg), ciprofloxacin (5Fg), tetracycline (30Fg), and cotrimoxazole (25Fg) (Oxoid Ltd). The diameters of inhibition zone around the discs were measured and interpreted as sensitive, intermediate or resistant as per the guideline set by Bauer, et al Bauer et al., 1966. Reference strains: E. coli ATCC 25922, S. pneumonia ATCC 49619,N. meningitides ATCC CDC327,H. influenza ATCC 49766 and S. aureus ATCC 25923 were tested as controls according to the National Committee for Clinical Laboratory Standards (NCCLS)National‐Committee‐for‐Clinical‐ Laboratory‐Standards, 1979. Lastly, data analysis was conducted using the SPSS statistical software (Version 17) and presented using a descriptive statistical method such as tables and graphs.
Results
Three thousand and eighty‐five (3,085) suspected meningitis cases were examined using culture in microbiology laboratory. Of whom 1589, (51.5%) were male. Over half of 3,085, (69 %) of the cases were less than 14 years of age and mean age was 2.87+ 0.99 SD year.
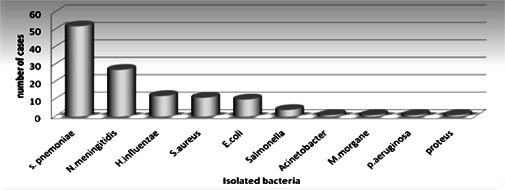
Bacterial pathogens were isolated from 120 patients showing an isolation rate of 3.8 %. No mixed bacterial infection was observed. The common isolated pathogen was S. pneumoniae 52 (43.3 %), N. Meningitidis 27 (22. 5 %),H. influenzae 12 ( 10 %) ( Figure 1 ).
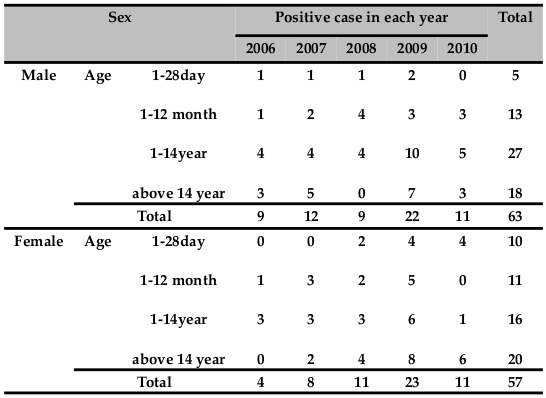
From the total of 120 cases 63(52.5 %) were male, while 57(47.5 %) were female. Sex ratio of positivity for bacterial meningitis between male to female was 1:1.1. There is no statistical significant association with isolated bacteria and sex. (Chi‐Square =0.49, P value = 0.824). Majority of the isolate (68.3%) of bacterial meningitis occurred in pediatric age groups. No statistical significant association was found between isolated bacteria and age (Chi‐Square =0.208, P value = 0.976) ( Table 1 ).
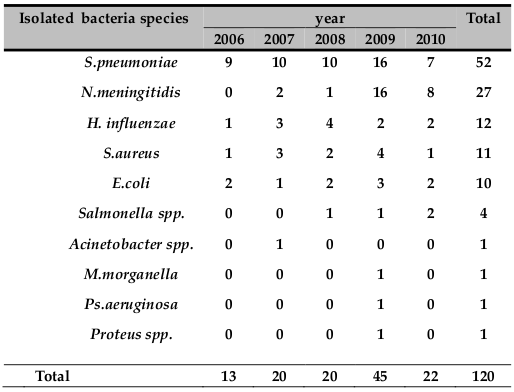
The numbers of bacterial meningitis cases in each year from 2006 to 2010 were 13, 20, 20, 45 and 22, respectively. Forty‐five (37.5%) of bacterial pathogen were isolated only in the year 2009 ( Table 2 ).
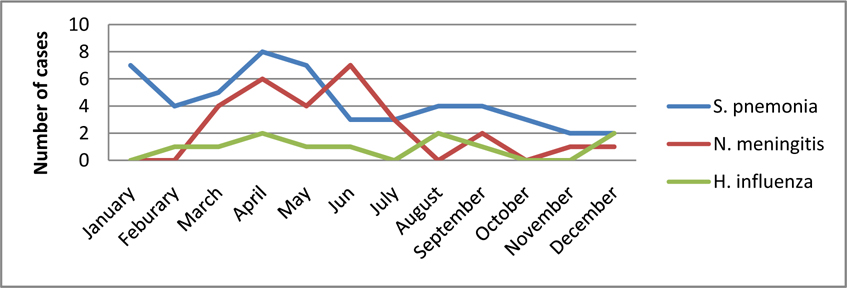
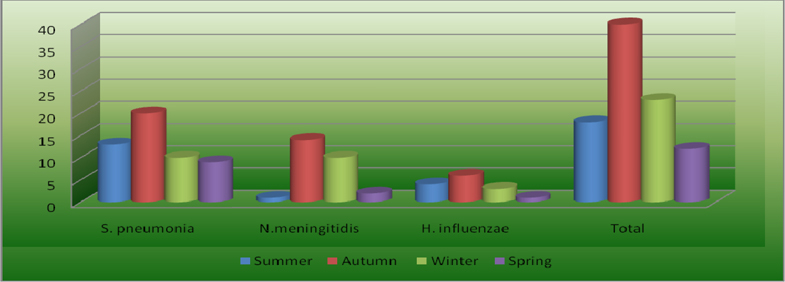
A seasonal variation of S. pneumonia meningitis was observed in this study. The majority of cases 31 (60 %) were isolated between January and May presenting with a two‐peak pattern. One peak was noticed during summer in January, and a second occurred during autumn, in April and May.
Again seasonal variation of M. meningitis was observed in the study. The majority of cases 24 (88.8 %) were isolated between March and [un presenting with a two-peak pattern. One peak was noticed during autumn, in April, and a second occurred during late autumn and early summer in May and Jun.
No seasonal variation was observed on the case of H. influenzae rather responsible for a relatively stable proportion of bacterial meningitis cases throughout the five years ( Figure 2 ).
Rate of bacterial mcningitidis isolation was relatively high during autumn seasons in March, April and May, were 4.8%, 6.2% and 4.2%, respectively. Whereas it was low during spring in October and November it was 1.5% each ( Figure 3 ).
Uniform antibiogram was used during the study period for all specimens so ciprofloxacin, penicillin, amoxicillin, cotrimoxasolc, ampicillin, gentamicin, erythromycin, chloramphenicol and tetracycline were the antibiotics that demonstrated in-vitro activity regularly against all cultured organisms.
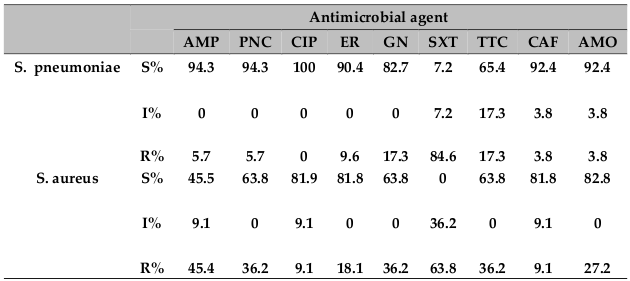
Sixty-three gram-positive bacteria were isolated from cerebrospinal fluid. Except three of all the 52 (94.2%) isolates of S. pneumoniae were sensitive to penicillin. The susceptibility patterns for nine antimicrobial agents are presented in Table 3 .
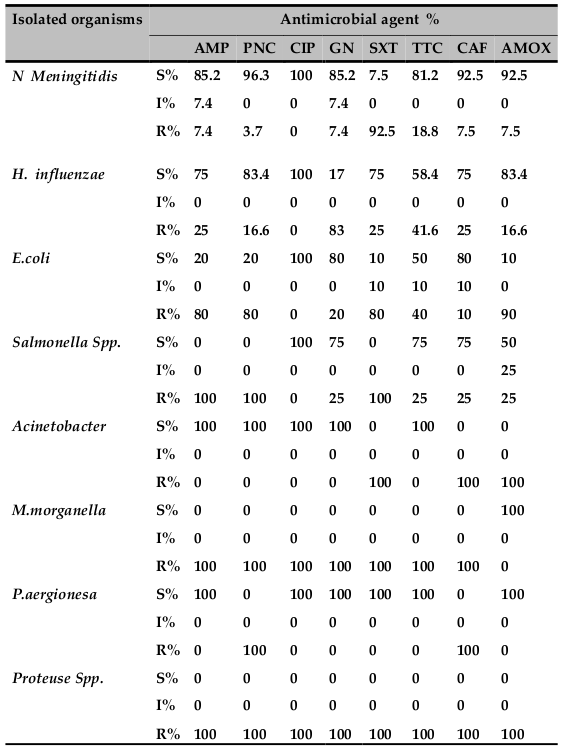
All isolated N.meningitis and all isolated H. influenzae were sensitive to ciprofloxacin. Whereas E. coli was found to be resistant to all antibiotics except for ciprofloxacin. Other gram‐negative bacilli other than H. influenza) showed a high percentage of resistance to several common drugs tested. The susceptibility patterns for seven antimicrobial agents are presented in Table 4 . In general ciprofloxacin was the most effective drugs against the tested gram‐positive and gramnegative bacteria.
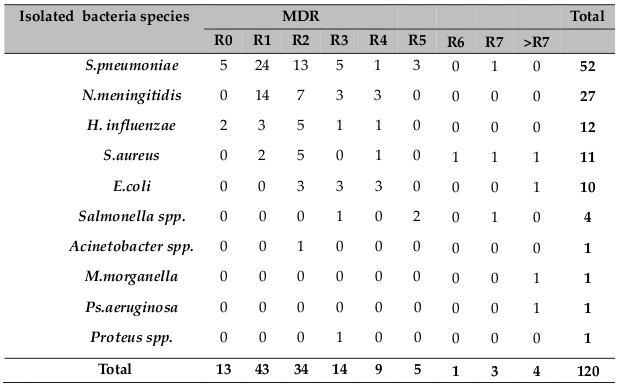
Multiple resistance (resistance to two or more drugs) was observed in 70 (58.3%) of the Isolates. Among the gram positives, multi drug resistance was observed in S. aureus, while among the gram negatives, different spp. were observed and presented in Table 5 .
Discussion
Acute bacterial meningitis is a medical emergency, which deserve early diagnosis and aggressive therapy. Most often therapy for bacterial meningitis has to be initiated before the etiology is isolated. The choice of initial antimicrobial therapy in community acquired acute bacterial meningitidis (CAABM) is based on the most common pathogen prevalent in a particular geographical area and age group and its antibiotic sensitivity pattern WHO, 1998.
Though the common pathogens associated with CAABM are S. pneumoniae, H. influenzae and N. meningitidis, the etiological agents and their relative frequency may vary in different geographical areas. Some changing trends in the epidemiology of CAABM have also been reported worldwide over the past few decadesNorheim et al.,2006Theodoridou et al., 2007. In this retrospective study the three major organisms responsible for bacterial meningitis were S.pneumoniae (43.3%), N. meningitis (22.5%) and H. influenzae (10%). similar trend reported from North parts of YemenAl Khorasani and Banajeh, 2006.
S. pneumonia remains the leading etiological agent of community acquired acute bacterial meningitis (CAABM) over the study period both in adults and children. Accounting for 52 (43.3%) of the total cases in our study, whereas a similar trend reported from Addis AbabaMuhe and Klugman, 1999, Madagascar Migliani et al.,2002, and Northern parts of Yemen Al Khorasani and Banajeh, 2006. In contrast to the above observation, some studies in Addis Ababa Hailu and Muhe, 2001 and in South Western Uganda, Kiwanuka and Mwanga, 2001 report that Hib to be the most common cause of bacterial meningitis among children, probably this was the period before the introduction of Hib vaccines. Other study in Belgium Hoeck et al., 1997 report meningococcal meningitis to be the major cause of meningitidis. In this retrospectives study S. pneumonia were isolated in all age group but, more than (64.3 %) of the case was pediatric age groups, reflecting a similar trend reported from Antananarivo, MadagascarMigliani et al., 2002, Malaysia Erleena et al., 2008 and Indian studies have also reported a high incidence of pneumococcal meningitis in children Mani et al., 2007. The mean age affected by S. pneumonia meningitis was 9.1 years.
The majority of S. pneumonia (60 %) was isolated between January and Jun presenting with two‐peak pattern. One peak was noticed during summer in January, and a second occurred during autumn, in May and Jun. This finding reflects a similar trend reported from Greece Theodoridou et al., 2007 ( Figure 2 ).
In our study only three isolates of S. pneumonia were resistance to penicillin some study in Ethiopia have reported the emergence of penicillin resistance Muhe and Klugman, 1999Mulu et al., 2005. This also true in Indian study Mani et al., 2007. So there is a need for continued monitoring for penicillin resistance in pneumococcal isolates, because at present it is a significant problem in Ethiopia.
N. meningitidis was found to be the second cause of childhood bacterial meningitis in the present study. Twenty‐seven (22.5%) cases of meningococcal meningitis were detected in the last five years. More than 60% meningococcal meningitis cases of the study were isolated in 2009. This study reiterates the finding of a low prevalence of meningococcal meningitis except during epidemics, in various study in East Africa: EthiopiaMuhe and Klugman, 1999, UgandaKiwanuka and Mwanga, 2001, KenyaAkpede et al., 1994and IndianMani et al., 2007. The majority of (88.8 %) was isolated between March and Jun presenting with two‐peak pattern. One peak was noticed during autumn, in April, and a second occurred during in May and Jun ( Figure 2 ) this is may be due to a dry season which is characterized by dust wind and upper respiratory tract infection due to cold night and at the same time the local immunity of the pharynx is diminished this lead to a high risk of meningococcal meningitis. This finding is in line with the previous reports from Gondar University Hospital Mengistu et al., 2003 and Greece Theodoridou et al., 2007.
Most cases due to N. meningitidis occurred in infants and young children: 18.5% occurred in infants less than 1 year and 81.5% in children less than 14 years of age. The mean age of N. meningitidis cases approximately was 3.9 years. In contrast to the above finding a study from Gondar UniversityMengistu et al., 2003, Addis Ababa Kristos and Muhe, 1993, the mean age were relatively higher because the majority (80%) of the cases were elderly groups between (15‐30) years.
Probably these had been due to both researches done during an epidemic year on meningococcal meningitis in the North Gondar and Addis Ababa respectively. But the data derived from our studies agree with the findings reported in Athens Theodoridou et al., 2007.
In this study H. influenzae was responsible for a relatively stable proportion of bacterial meningitis cases throughout the five years. All 12 (10%) cases of meningitis occurred in pediatric age groups. Majority, 11 (91.6%) H. influenzae meningitis were isolated from both neonate and infant age groups.
The mean age of children affected by Hib meningitis was 0.7 years, quite lower compared to the age of children affected by N. meningitidis and S. pneumoniae. The 16.6% and 91.6 % of children affected by Hib meningitis were aged < 28 day and 1 year respectively. Reflecting a similar trend reported from Addis Ababa Hailu and Muhe, 2001 and Uganda which has a high incidence of H. influenzae meningitis in the pediatric age group Kiwanuka and Mwanga, 2001. Some Indian authors have also reported a high incidence of H. influenza meningitisMani et al., 2007.However, an earlier study from Gondar University HospitalMulu et al., 2005 shows that the incidence H. influenzae meningitis was zero this is probably in the previous time human blood is commonly used for making both blood and chocolate agar. A seasonal variation in Hib meningitis cases was not observed.
S. aureus werethe predominate isolated gram positive bacteria next to S. pneumonia. Eleven (9.2%) of the case were S. aureus. This finding is in line with the research conducted from University of Gondar Teaching HospitalAseffa and Yohannes, 1996 and other research from West Africa, NigeriaAkpede et al., 1994. Resistance to the common antibiotics tested was high and the majority (72.2%) of S. aurous was isolated from the elderly (above 14 years).
Ten (8.3%) cases of meningitis were due to E. coli. More than 87% isolate of E. coliwas found to be resistance to multiple drugs such as penciling, ampicillin, tetracycline, gentamicin and cotrimoxazole and the data derived from our studies agree with the findings reported from University of Gondar Teaching Hospital Aseffa and Yohannes,1996 the majority case were isolated from pediatric age groups. Salmonella species were found to be susceptible to ampicillin, gentamicin, chloramphenicol and ciprofloxacin.
Acinetobacter 1 cases, (0.8%), M. morgane 1 case, (0.8%), P. aeruginosa 1 case, (0.8%) and 1 cases of meningitis due to proteus specieswere isolated asimportant pathogens of community‐acquired as well as nosocomial meningitis especially among the elderly and in patients with chronic diseases. one case of M. morgane, and one caseP. aeruginosa were isolated in this study were not encountered in earlier studies from University of Gondar Teaching Hospital Mulu et al., 2005 but no case of L. monocytogenes meningitis was seen in this study though the incidence is low in most East African studyKiwanuka and Mwanga, 2001, Migliani et al., 2002 and Al Khorasani and Banajeh, 2006, it should be considered especially in the elderly and immunocompromised patients, since it is known to be resistant to third generation cephalosporins used in the empirical treatment of bacterial meningitis.
The small number of antibiotic discs such as ceftraxon, nalidixic acid, and norfloxacin were not available regularly in the period between 2006‐2010. So, no uniform for all specimens made analysis of results of antibiotic sensitivity impossible. However it is worth noting that ciprofloxacin, penicillin, amoxicillin, cotrimoxasole, ampicillin, gentamicin and tetracycline were the antibiotics that are available routinely in the study paired (2006 ‐ 2010).
The antimicrobial therapy of bacterial meningitis has come under increasing inspection owing to numerous reports of antimicrobial resistance by the common causative organismsAseffa and Yohannes, 1996Muhe and Klugman, 1999. This may be a reflection of unregulated use of antibiotics particularly in developing country.
Conclusion
In conclusion S. pneumonia constituted the leading cause of bacterial meningitis in all age groups during the five years study and more than 68.3% of the cases were less than fourteen years old and the majority of cases 31 (60 %) were isolated between January and May presenting with a two peak pattern and only three isolates of S. pneumonia were resistance to penicillin. N. meningitidis was the second common cause of bacterial meningitis. Variations in the incidence of meningococcal meningitis were presented over time. The majority of cases (88.8 %) were observed between March and June presenting with a two peak pattern. One peak was noticed during autumn, in April, and a second occurred during May and June following the well described natural cycles that characterize the epidemiology of meningococci and 81.5% were isolated in pediatric age groups. H. influenzae type b was an important cause of bacterial meninigites and relatively stable proportion of cases throughout five years. More than 91.6 % of H. influenzae were isolated from both neonates and infants. The frequency of single as well as multiple drug resistance is alarmingly high.
The limitation of this study was a small number of cephalosporin antibiotic discs such as ceftraxon, cefatazidin and cefalaxin. And other quinoline antibiotic discs such as nalidixic acid and norfloxacin were not available regularly in the period between 2006‐2010, no uniform antibiotics disk used for all specimens so analysis of results of antibiotic sensitivity is impossible.
References
-
O.
Akpede,
P.
Abiodun,
M.
Sykes,
C.
Salami.
Childhood bacterial meningitis beyond the neonatal period in southern Nigeria: changes in organisms/antibiotic susceptibility. East African medical journal.
1994;
71
:
14-20
.
-
A.
Al Khorasani,
S.
Banajeh.
Bacterial profile and clinical outcome of childhood meningitis in rural Yemen: a 2-year hospital-based study. Journal of Infection.
2006;
53
:
228-234
.
-
A.
Aseffa,
G.
Yohannes.
Antibiotic sensitivity pattern of prevalent bacterial pathogens in Gondar, Ethiopia. East African medical journal.
1996;
73
:
67-71
.
-
A.W.
Bauer,
W.M.
Kirby,
J.C.
Sherris,
M.
Turck.
Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol.
1966;
45
:
493-496
.
-
M.
Cheesbrough.
Details of Part 1. In District Laboratory Practice in Tropical Countries. Cambridge University Press (CUP).
2006;
:
380-380
.
-
J.
Commey,
O.
Rodrigues,
F.
Akita,
M.
Newman.
Bacterial meningitis in children in southern Ghana. East African medical journal.
1994;
71
:
113-117
.
-
H.
Erleena,
I.
Jamaiah,
M.
Rohela.
Bacterial meningitis: a five year retrospective study at university of Malaya Medical Center. Department of Parasitology, Faculty of medicine. South east Asian Journal of tropical medical and public health.
2008;
39
:
73-77
.
-
M.
Hailu,
L.
Muhe.
Childhood meningitis in a tertiary hospital in Addis Ababa: clinical and epidemiological features. Ethiopian medical journal.
2001;
39
:
29-38
.
-
K.J.V.
Hoeck,
L.M.
Mahieu,
M.H.
Vaerenberg,
K.J.V.
Acker.
A retrospective epidemiological study of bacterial meningitis in an urban area in Belgium. European Journal of Pediatrics.
1997;
156
:
288-291
.
-
M.
Jawetz.
Adelbergis Medical Microbiology. In Antibacterial and Antifungal chemotherapy (Prentice-Hall International Inc).
.
-
J.P.
Kiwanuka,
J.
Mwanga.
Childhood bacterial meningitis in Mbarara Hospital, Uganda: antimicrobial susceptibility and outcome of treatment. African health sciences.
2001;
1
:
9-11
.
-
T.G.
Kristos,
L.
Muhe.
Epidemic meningococcal meningitis in children. A retrospective analysis of cases admitted to ESCH (1988). Ethiopian medical journal.
1993;
31
:
9-14
.
-
R.
Mani,
S.
Pradhan,
S.
Nagarathna,
R.
Wasiulla,
A.
Chandramuki.
Bacteriological profile of community acquired acute bacterial meningitis: a ten-year retrospective study in a tertiary neurocare centre in South India. Indian journal of medical microbiology.
2007;
25
:
108
.
-
G.
Mengistu,
K.
Mitiku,
W.
Teferi.
Analysis and reporting of meningococcal meningitis epidemic in north Gondar 2001-2002. Ethiopian medical journal.
2003;
41
:
319-331
.
-
M.R.F.
Meningococcal disease.
.
-
R.
Migliani,
J.
Clouzeau,
J.W.
Decousser,
N.
Ravelomanana,
J.
Rasamoelisoa,
H.
Rabijaona,
J.A.
Dromigny,
P.
Pfister,
J.F.
Roux.
Les méningites bactériennes non tuberculeuses de l’enfant à Antananarivo, Madagascar. Archives de Pédiatrie.
2002;
9
:
892-897
.
-
L.
Muhe,
K.P.
Klugman.
Pneumococcal and Haemophilus influenzae meningitis in a children's hospital in Ethiopia: serotypes and susceptibility patterns. Tropical Medicine & International Health.
1999;
4
:
421-427
.
-
A.
Mulu,
A.
Kassu,
B.
Tassema.
Bacterial isolates from cerebrospinal fluids and their antibiotic susceptibility patterns in Gondar University Teaching Hospital, Northwest Ethiopia. Ethiopian Journal of Health Development.
2005;
19
.
-
National-Committee-for-Clinical-Laboratory-Standards.
Performance standards for antimicrobial disc susceptibility tests. In Approved Standard, ASM-2 (NCCLS, Villano-van, pa. ).
1979
.
-
G.
Norheim,
E.
Rosenqvist,
A.
Aseffa,
M.A.
Yassin,
G.
Mengistu,
A.
Kassu,
D.
Fikremariam,
W.
Tamire,
E.A.
Hoiby,
T.
Alebel.
Characterization of Neisseria meningitidis Isolates from Recent Outbreaks in Ethiopia and Comparison with Those Recovered during the Epidemic of 1988 to 1989. Journal of Clinical Microbiology.
2006;
44
:
861-871
.
-
H.E.
Nur,
I.
Jamaiah,
M.
Rohela,
V.
Nissapatorn.
Bacterial meningitis: A five year (2001-2005) retrospective study at University Malaya Medical Center (UMMC), Kuala Lumpur, Malaysia. 2008
.
-
M.N.
Theodoridou,
V.A.
Vasilopoulou,
E.E.
Atsali,
A.M.
Pangalis,
G.J.
Mostrou,
V.P.
Syriopoulou,
C.S.
Hadjichristodoulou.
Meningitis registry of hospitalized cases in children: epidemiological patterns of acute bacterial meningitis throughout a 32-year period. BMC Infect Dis.
2007;
7
:
101
.
-
WHO.
Basic laboratory procedures in clinical bacteriology. In (WHO, Geneva, Switzerland).
1991
.
-
WHO.
Control of epidemic meningococ-cal diseases. In WHO Practical Guidelines (Geneva Switzerland).
1998
.
Comments

Downloads
Article Details
Volume & Issue : Vol 2 No 05 (2015)
Page No.: 270-278
Published on: 2015-05-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7382 times
- Download PDF downloaded - 1918 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress