Abstract
Introduction: Psoriasis is a common chronic inflammatory disease that affects the physical, mental and sexual well-being of patients. Numerous side effects of different treatments and inadequate response to medications have resulted in pursuit of ideal treatment with low toxicity in low burden psoriasis hence complementary medicine. This study aims to evaluate the effects of whey with dodder oxymel on mild to moderate psoriatic skin lesions.
Methods: A 12-week double-blind, randomized, controlled, clinical trial was designed. Ninety psoriatic patients participated in the intervention. Drug and placebo were randomly assigned to two groups identically (whey with dodder oxymel and lactose). Patients were visited twice by a dermatologist. Their clinical responses were evaluated using the Psoriasis Area Severity Index (PASI), the Dermatology Life Quality Index (DLQI), the Visual Analogue Scale (VAS) and the Body Surface Area (BSA).
Results: After 12 weeks, in the intragroup analysis, the mean PASI score (P-value < 0.001) and BSA (P-value = 0.004) decreased in the intervention group. The mean VAS score (P-value < 0.001) and DLQI (Pvalue < 0.001) in both groups decreased. However, this decrease was much higher in the intervention group. In the intergroup analysis, 70% of patients reported improvement in PASI score (P-value < 0.001), the 88% improvement in quality of life (P-value < 0.001) and pruritus intensity (VAS) (P-value < 0.001), and the 54% reduction was detected in the area of lesions (BSA) (P-value = 0.001) as compared to the placebo group.
Conclusion: It appears that whey with dodder oxymel would improve psoriasis conditions and it can increase patients’ quality of life.
Background
Psoriasis is a common chronic, genetic and immune-mediated disease, which affects skin, joints, and nails. It has a negative impact on the social, economic, sexual, and psychological lives of the patients 123. The prevalence rate of psoriasis is 2–3% 1. It is associated with severe medical comorbidities such as cardiovascular diseases, metabolic syndrome, depression, osteoporosis, psoriatic arthritis, nephrotoxicity, immunological diseases, e.g. inflammatory bowel diseases and rheumatoid arthritis, and even malignancy 4.
There is no definitive cure for psoriasis, but multiple suppressive symptomatic therapies are currently used, which have many undesirable side effects 3. These therapeutic methods include topical agents such as emollients, topical corticosteroids, systemic medicaments, e.g., immunosuppressives and biologic drugs, phototherapy and psoralen therapy. Long-term use of these treatments may impose various complications on the patients, e.g., skin atrophy, nephrotoxicity, cirrhosis, liver fibrosis, hyperlipidemia, higher chances of hospitalization due to hazardous infections and increased risk of skin cancers 567. Still, from last more than a decade, curative management of moderate to severe psoriasis hypothesizing role of Group A Streptococci in the sole causation of psoriasis and encouraging results with long-term antibiotic against GAS should be considered 8910.
The chronic condition, various complications and patients' dissatisfaction with these conventional therapies, has led the patients to use alternative therapies in recent years 111213. CAM methods, such as traditional medicines (TM), homeopathy, ichthyotherapy and spa therapy have been used in different studies, and they have shown improvements in symptoms and patients' satisfaction 141516.
Persian medicine (PM) is one of the CAM branches, which roots back to over 8000 years ago. In PM, treatments are based on four models, i.e., lifestyle modification, diet-o-therapy, pharmacotherapy and physical therapy 17. Whey that can be categorized in both diet-o-therapy and pharmacotherapy group is a functional food that has been prescribed by many famous ancient Persian physicians. Avicenna and Rhazes (Muhammad ibn Zakariya al-Razi) applied whey for its gentle laxative and excellent moisturizing property to treat various diseases such as gout, rheumatism, neurological diseases, e.g., melancholia and skin diseases 18.
In ancient manuscripts, whey was prescribed individually or in combination with herbal preparations like their aqueous extracts or oxymels. Cuscuta seeds are one of these famous herbs. There are different Cuscuta species in the world. One of them is Giant dodder with the scientific name of Cuscuta reflexa which is traditionally called Aftimoun in Iranian herbal market. This herb contains several chemical elements such as flavonoids (kaempferol, quercetin), dulcitol, bergenin, coumarins, glycosides and lactones. These active components have antioxidant, antiviral, antibacterial, antipruritic, anti-inflammatory, anti-allergic, diuretic and laxative effects, which have been proved in various studies 1920.
Given the availability, low cost, low complications and effectiveness of whey in treatment of other skin diseases and the benefits of Cuscuta reflexa 18, we aimed to assess the efficacy of oral administration of whey combined with dodder oxymel (WwDO) in the treatment of mild to moderate skin lesions of psoriasis in a randomized double-blind, placebo-controlled trial.
Methods
Trial design
This study was a two-armed double-blind, randomized, placebo-controlled clinical trial using a parallel design.
Patients
From November 2015 to June 2016 Ninety patients, who were referred to the Skin and Stem Cell Research Centre (SSRC) dermatology clinics of Tehran University of Medical Sciences, in Iran were assessed for eligibility criteria for being included in the study. The eligibility evaluation was performed by two dermatologists. The inclusion criteria for the study were: age of more than 18 years, diagnosis of mild to moderate psoriasis by the dermatologist, diagnosis of controlled psoriasis vulgaris (no change in the last two months regarding severity and area of the lesions), and involved body surface less than 10%.
Exclusion criteria were:
1) pregnancy and lactation,
2) use of psoralen and UVB in the past 30 days,
3) Patients with other types of psoriasis, such as pustular, erythrodermic, palmoplantar,
5) psoriatic arthritis,
6) having a history of lactose susceptibility,
7) kidney stones,
8) using psoriasis-resonating agents, such as beta-blockers, non-steroidal anti-inflammatory drugs, calcium channel blockers, interleukins, and lithium,
9) suffering from uncontrolled cardiovascular, respiratory, hematologic or urinary disorders.
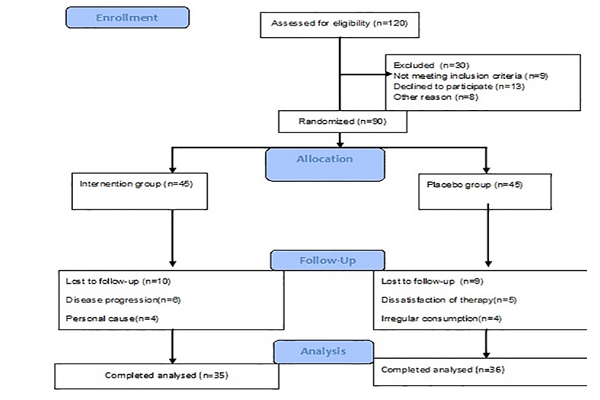
90 patients met our eligibility criteria and were assigned to intervention and control groups– WwDo group and Placebo group. Demographic and clinical characteristics such as name, address, and contact number, age, sex, duration of the disease, history of psoriasis in the family, weight, and height- after which the BMI was estimated- of all participants were recorded at the beginning of the study Figure 1.
Drug and Placebo preparation
WwDo
The mentioned medicine in this clinical trial is a traditional Persian preparation and has been certified and approved by its manufacturer named NIAK pharmaceutical company (Registration code: c24164).
Placebo
About 100 gm of sugar and 200 gm of pharmaceutical lactose were combined as homogenous powders and poured into 300-gm cans.
Interventions
Both intervention and placebo drugs were in a powdery formation, which was packed in similar shape, weight, and color cans. The drugs were randomly assigned to the two groups. All the patients were instructed to dissolve 10 grams of the drug in glass of water to drink in morning fasting for a period of 12 weeks. Also, both the groups were advised to continue using an emollient ointment _20% glycerine based on Eucerin_ and coal tar shampoo. All the patients were visited twice during the study (at the beginning and the end of the 12th week). In each visit a photo was taken from the patient with a Canon EOS 400D camera_ the camera had an EFS18-55mm lens and a sensor accuracy of 10 megapixels. These photos were attached to the patients’ files.
Outcome assessment
The severity of disease was the first outcome measure of this study which was evaluated by PASI score. The Psoriasis Area and Severity Index (PASI) score assesses the severity and extent of the disease by measuring the sum of the redness, thickness, and scaliness of the lesions (each on a 0–4 scale) and weights these by the area of involvement (measured on a scale of 1–6 ). Score for PASI ranges from 0 (no disease) to 72 (severe disease). More than 50% reduction in PASI score is considered a significant improvement 21. Body surface area (BSA) and Dermatology life quality index were our secondary and tertiary outcome measurements. The BSA (palm ratio=1%) was used to assess the affected area of the lesions. The head and neck were set at 10%, the upper limb at 20%, the trunk at 30%, and the lower limb at 40% 2223. The DLQI _the Persian version_ was used to assess the quality of life. This was a ten-item questionnaire for assessing symptoms and feelings, daily activities, leisure, work, education, and social communication of patients. The DLQI score ranges from 0 (no impairment) to 30 (maximal impairment) 24. The VAS index was the other outcome measurement which was used to assess the severity of the pruritus. The VAS index ranges from 0 (no pruritus) to 10 (very severe pruritus) 25. The Common terminology criteria for Adverse Event (CTCAE) V4.0 was used to evaluate possible gastrointestinal and dermatological complications 26. All outcome measurements were evaluated in the baseline and at the end of the study in the 12th week, except the CTCAE which was only assessed at the end of the study.
Sample size
Based on the statistical calculations and the same studies (e.x., Poulin et al.) 27, the sample size was estimated by considering one-sided significance level of 0.05, a power of 0.90, a confidence interval of 0.95, an effect size of 0.75 and a probable 20% drop-out rate to be 45 patients in each group.
Randomization and blinding
Ninety patients were randomly allocated to WwDO and placebo groups in a parallel design. One of the clinic secretaries was trained to use a randomized list for allocating the patients. The randomization list_ non-stratified, with the same block lengths _ was generated by using Excel Microsoft. The physicians, researchers, and statistician were blind to the allocation of the patients. Moreover, the patients were blind to the drug allocation because of the similarity of the drug cans in shape, colour, and weight.
Statistical analysis
The SPSS software (version.20, IBM Corporation) was used to analyze the data. Normality of continuous variables was assessed using the Kolmogorov–Smirnov test. The Chi-square test, the independent and paired t-test were used for statistical analysis. P-values less than 0.05 (P<0.05) were considered to be statistically significant. Demographic and clinical characteristics of the patients were shown as the mean ± standard deviation for continuous variables.
Ethical considerations
This study complied with the Declaration of Helsinki (1989 revision) and was approved by the Local Medical Ethics Committee of the Tehran University of Medical Sciences (TUMS) with reference number: IR. TUMS. REC.1394.859 and registered in the Iranian Registry of Clinical Trials (registration ID: IRCT2015100524359N1). All of the participants signed an informed consent form before enrollment in the study.
Results
From November 2015 to June 2016 a total of 120 patients were assessed for eligibility criteria. Finally, 90 of them who met the criteria and signed their written informed consent were randomly assigned to WwDO and placebo groups, 45 patients in each group. After beginning the study ten patients in the WwDO group (four due to their disease progression and six due to personal causes) and nine patients in the placebo group (five due to the dissatisfaction of the treatment and four due to irregular consumption of the drug) were lost to follow up. Eventually, a total of 71 patients (35 in the WwDOgroup and 36 in the placebo group) continued the treatment for 12 weeks. Flowchart of the patients’ allocation, enrollment, intervention, and follow up is shown in, Figure 1. Baseline demographic and the clinical characteristics of participants are shown in Table 1.
| Variables | WwGD Group | Placebo Group | P value | ||
| N* | P ** | N | P | ||
| Sex | F=16 | 45.7% | F=21 | 58.3% | 0.29 |
| M=19 | 54.3% | M=15 | 41.7% | ||
| Mean±SD *** | Mean±SD | ||||
| Age | 40.88±11.76 | 38.33±11.64 | 0.36 | ||
| BMI | 26.99±4.58 | 25.51±4.63 | 0.18 | ||
| PASI score | 5.48±4.49 | 4.38±2.32 | 0.2 | ||
| BSA | 4.68±4.34 | 3.89±2.70 | 0.36 | ||
| VAS | 5.68±2.27 | 5.86±2.89 | 0.78 | ||
| DLQI | 10.94±7.18 | 7.97±5.15 | 0.05 | ||
Intergroup Analysis
The independent t-test showed that the mean scores of dependent variables (PASI score, BSA, VAS, and DLQI) before the intervention were not significantly different in the two groups (Table 1). There were no significant differences (P>0.05) between the two groups in the baseline demographic and clinical characteristics. The results of the independent t-test showed that the PASI scores decreased in each group at the end of the study period, but this decrease in the WwDO group was more noticeable (P-value < 0.001). The BSA scores showed a satisfactory decrease in the affected area of the lesion in the intervention group but increased in the placebo group (P<0.01). The VAS scores (P-value < 0.001) and DLQI (P-value < 0.001) decreased in each group, but it was more significant in the WwDO group. Furthermore, the results of the covariance analysis showed that the mean scores of dependent variables (PASI score, VAS, BSA, and DLQI) after the intervention were higher in the control group (Table 2 and Figure 2).
| Variable | Week 0 | Week 12 | ||||
| Intervention Mean±SD | PlaceboMean±SD | P value | Intervention Mean±SD | PlaceboMean±SD | P value | |
| PASI score | 5.48±4.49 | 4.38±2.32 | 0.2 | 2.43±2.64 | 4.25±2.44 | <0.001 |
| BSA | 4.68±4.34 | 3.89±2.70 | 0.36 | 2.69±2.62 | 4.07±2.78 | 0.001 |
| VAS | 5.68±2.27 | 5.86±2.89 | 0.78 | 1.80±1.89 | 4.25±2.77 | <0.001 |
| DLQI | 10.94±7.18 | 7.97±5.15 | 0.05 | 3.42±3.36 | 6.83±5.01 | <0.001 |
Intragroup Analysis
The result of the paired t-test showed that the mean PASI score decreased from 5.48±4.49 to 2.43±2.64 (P-value < 0.001), BSA reduced from 4.68±4.34 to 2.69±2.62 (P-value = 0.004), the mean VAS score lessened from 5.68±2.27 to 1.80±1.89 (P-value < 0.001) and DLQI decreased from 10.94±7.18 to 3.42±3.36 (P-value < 0.001) after 12 weeks in intervention group (Table 3 and Figure 3).
| Variable | Week 0 | Week 12 | P-Value | Week 0 | Week 12 | P-value |
| Intervention (Mean± SD) | Intervention (Mean± SD) | Placebo(Mean± SD) | Placebo(Mean± SD) | |||
| PASI score | 5.48±4.49 | 2.43±2.64 | <0.001 | 4.38±2.32 | 4.25±2.44 | 0.63 |
| BSA | 4.68±4.34 | 2.69±2.62 | 0.004 | 3.89±2.70 | 4.07±2.78 | 0.37 |
| VAS | 5.68±2.27 | 1.80±1.89 | <0.001 | 5.86±2.89 | 4.25±2.77 | 0.001 |
| DLQI | 10.94±7.18 | 3.42±3.36 | <0.001 | 7.97±5.15 | 6.83±5.01 | 0.03 |
In the placebo group, the mean PASI score changed from 4.38±2.32 to 4.25±2.44 (P-value = 0.63), BSA increased from 3.89±2.70 to 4.07±2.78 (P-value = 0.37), VAS decreased from 5.86±2.89 to 4.25±2.77 (P-value = 0.001), and DLQI decreased from 7.97±5.15 to 4.25±2.77 (P-value = 0.03) after 12 weeks, (Table 3 and Figure 3).
Adverse effects
During the study, eight patients (23%) in WwGD group suffered from gastrointestinal problems, such as the after-meal bloating and heaviness in the stomach which was controlled with a reduction in the drug dosage and using a carminative in few days. In the placebo group, four patients (13%) suffered from flashing and redness of the lesions after two weeks using the drug. Discontinuation of the drug for three days and then restarting with a lower dose was recommended. Two of them gradually improved, while the other two who did not respond to this strategy were excluded from the study.
Discussion
Psoriasis is a common chronic immune-mediated disease created by genetic aberrations in keratinocytes 28. With the activation of keratinocytes, Th1 pathway cytokines, i.e., interferon-c [IFN-c], tumor necrosis factor-a [TNF-a], and interleukin-2 [IL-2] have been perceived in its plaques and in the peripheral circulation which are causing keratinocytes hyper-proliferation. In addition to, insulin-like growth factor [IGF] stimulating keratinocyte proliferation increases in this disease, and transforming growth factor-β [TGF-β] inhibiting keratinocyte proliferation decreases in psoriasis 2829.
The imbalance between oxidants and antioxidants, which leads to reduction endogenous antioxidant enzymes, e.g., glutathione peroxidase (GP), and increase oxidative stress markers, e.g., malondialdehyde (MDA) is another theory that has been mentioned for the pathogenesis of the disease. The increased risk of oxidative stress-mediated diseases such as cardiovascular disorders and diabetes mellitus associated with psoriasis reinforces this theory30.
Due to the chronicity of the disease and the adverse effects of conventional medications, many clinical trials have been conducted with harmless, beneficial, and inexpensive treatments 111231.
This study was designed as the first clinical trial in Iran that prescribed whey with dodder oxymel for mild to moderate psoriasis. Based on the results, the severity, area, depth of the lesion, and the pruritus intensity significantly decreased compared to the placebo group, thus improving patients’ life quality. About 70% of the patients reported significant improvement in the PASI score, the 88% improvement was observed in the quality of life and pruritus intensity (VAS), and the 54% reduction was detected in the area of the lesion (BSA) as compared to the placebo group.
Few data regarding the efficacy of whey protein on psoriasis are existing. Poulin et al. 27 assessed the efficacy of the two different dose regimens (5 and 10 gm/day) of XP-828L (a form of whey protein) in mild to moderate psoriasis for 8 and 16 weeks. At the endpoint, the area of the lesion (BSA) and the pruritus intensity (VAS) did not change, and reduction observed only in the PGA (P < 0.05). There were no significant differences between the two groups in the PASI score during the study. However, the PASI score reduction of 25% detected only in 9 patients in the intervention group.
Drouin et al. 32 determined the utility of XP-828L medication, used by Poulin, at a dose of 800 mg for 56 days.In the end, the PASI score reduction of 25% perceived in 38% of patients. DLQI showed to improve 0.9 ± 2.9 units and the pruritus intensity reduced by 31%.
Prussick et al. 33 prescribed a non-denatured whey protein to seven patients with moderate to severe psoriasis at a dose of 20 gm/day for three months. In the end, the PASI score showed to decrease by 50%.
The disadvantages of the study by Prussick et al. as compared to present study were the lack of a blinding process, the low number of patients, and the measurement of just one variable.
The limitations of this study concerning the studies mentioned above: 1) In the study of Poulin and Prussick, the patients were surveyed at three stages, whereas the patients of this study were assessed at only two stages due to lack of cooperation. 2) The dosage of medication was more in this study as compared to the study of Poulin and Drouin. 3) Due to the economic problems of the patients, routine tests performed in the studies of Poulin and Drouin were not carried out in our study.
The strengths of this study: (1) Therapeutic outcome encouraging for all variables. (2) Four variables analyzed. (3) Giant dodder used with whey protein, which probably improved symptoms by a higher margin than the other studies.
Whey protein is a functional food that has many protective and therapeutic effects on the human body, e.g., antioxidant and anti-inflammatory, anti-obesity, anticancer, blood pressure lowering, gut homeostatic, antidiabetic, osteoprotective, dermatoprotective, and immunomodulative. Its proteins and peptides ingredients are β-lactoglobulin, α-lactalbumin, immunoglobulin, lactoferrin, lactoperoxidase, glicomacropeptide, and bovine serum albumin. In addition, it is a rich source of branched-chain amino acids (leucine, isoleucine, and valine), and sulfhydryl amino acid cysteine a precursor of glutathione [34]. Glutathione (GSH) is an important endogenous antioxidant, and other antioxidants, such as Vitamin C, Vitamin E, and selenium need it for their functions. GSH also contributes to the treatment of other diseases caused by oxidative stress, such as cystic fibrosis, HIV, and other autoimmune diseases and cancers 33. Lactoferrin (Lf), the other antioxidant existing in the whey, has been proved to decrease immune cells’ productions, e.g., tumour necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-2, and IL-6 and interferon-gamma (INF-γ) and it has antibacterial and antiviral properties. Whey also contains TGF-β 2, a multifunctional cytokine which inhibits interleukin-2 (IL-2) production and regulates keratinocytes growth and function 323435. In addition, due to a high some of tryptophan amino acid, the whey protein increases serotonin level, and then reduces pruritus and inhibits depression 1836.
Also, apart from its effect on the heart, liver, kidney, and intestines, giant dodder (aftimon) has potent anti-inflammatory, anti-pruritus, and anti-allergic properties. It also has an inhibitory effect on insulin-like growth factor signalling pathway, which inhibits the hyperproliferation of keratinocytes1920373839. According to the results of this study, it seems that whey and dodder oxymel, with its additional effect on the immune system, reduction of inflammation, inhibition of cytotoxin secretion, increase in glutathione and serotonin, and subsequent reduction of depression, would improve psoriasis and increase the life expectancy of the patients.
Conclusions
In recent years, higher acceptance of complementary therapies and low-complication drug use have been observed in the society. In this study, whey protein with dodder oxymel was given to patients with mild to moderate psoriasis, and clinical improvement was observed. Given the beneficial effects of whey protein, it is recommended to carry out further studies using other anti-inflammatory drugs that may enhance the therapeutic results.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CCBY4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
BSA: Body Surface Area; CAM: Complementary and Alternative Medicine; CTCAE: Common Terminology Criteria for Adverse Events; DLQI: Dermatology Life Quality Index; GP: glutathione peroxidase; MDA: malondialdehyde; PASI: Psoriasis Area Severity Index; PGA: Physician Global Assessment; SSRC: Skin and Stem Cell Research Centre; TCM: Traditional Chinese Medicine; VAS: Visual Analogue Scale; WwGD: Whey Protein with Giant Dodder
Ethics approval and consent to participate
This study complied with the Declaration of Helsinki (1989 revision) and was approved by the Local Medical Ethics Committee of the Tehran University of Medical Sciences (TUMS) with reference number: IR. TUMS. REC.1394.859 and registered in the Iranian Registry of Clinical Trials (registration ID: IRCT2015100524359N1). All of the participants signed an informed consent form before enrollment in the study.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was conducted by the fund prepared by the school of the Persian Medicine, Tehran University of Medical Sciences, Tehran, Iran, for clinical researches. The financial sponsor of the project did not have any interference with these issues: in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Authors' contributions
Study conception, design and the definition of intellectual content: Atyabi, Shirbeigi, Mansori. Literature search: Atyabi, Eghbalian. Clinical study and experimental studies: Atyabi, Kordafshari. Acquisition of data: Atyabi, Eghbalian, kordafshari, Nejatbakhsh. Analysis and statistical analysis: Nasiri. Manuscript preparation, manuscript editing, and manuscript review: Atyabi, Eghbalian, Shirbeigi. Critical revision: Shirbeigi. All authors revised the paper and approved the final manuscript.
Acknowledgments
This study was derived from a Ph.D. thesis, entitled “An assessment of the effects of oral administration of whey protein on mild to moderate skin lesions of psoriasis: A double-blind randomized, placebo-controlled clinical trial “which was supported by the School of Persian Medicine, Tehran University of Medical Sciences, Iran, with special thanks to Dr. Sepideh Kolouri for her invaluable helps.
References
-
Prieto-Pérez
R.,
Cabaleiro
T.,
Daudén
E.,
Ochoa
D.,
Román
M.,
Abad-Santos
F..
Pharmacogenetics of topical and systemic treatment of psoriasis. Pharmacogenomics.
2013;
14
:
1623-34
.
-
Kimball
A. B.,
Jacobson
C.,
Weiss
S.,
Vreeland
M. G.,
Wu
Y..
The psychosocial burden of psoriasis. American Journal of Clinical Dermatology.
2005;
6
:
383-92
.
-
Kim
W. B.,
Jerome
D.,
Yeung
J..
Diagnosis and management of psoriasis. Canadian Family Physician Medecin de Famille Canadien.
2017;
63
:
278-85
.
-
Ni
C.,
Chiu
M. W..
Psoriasis and comorbidities: links and risks. Clinical, Cosmetic and Investigational Dermatology.
2014;
7
:
119-32
.
-
Lebwohl
M.,
Ting
P. T.,
Koo
J. Y..
Psoriasis treatment: traditional therapy. Annals of the Rheumatic Diseases.
2005;
64
:
ii83-6
.
-
Raut
G.,
Wairkar
S..
Management of psoriasis with nutraceuticals: an update. Complementary Therapies in Clinical Practice.
2018;
31
:
25-30
.
-
Smith
C. H.,
Anstey
A. V.,
Barker
J. N.,
Burden
A. D.,
Chalmers
R. J.,
Chandler
D. A.,
Chair of Guideline
Group.
British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. British Journal of Dermatology.
2009;
161
:
987-1019
.
-
Saxena
V. N.,
Dogra
J..
Long-term use of penicillin for the treatment of chronic plaque psoriasis. European Journal of Dermatology.
2005;
15
:
359-62
.
-
Saxena
V. N.,
Dogra
J..
Long-term oral azithromycin in chronic plaque psoriasis: a controlled trial. European Journal of Dermatology.
2010;
20
:
329-33
.
-
Dogra
L.,
Saxena
V.N.,
Dogra
J.,
Kishoria
N..
A controlled trial of oral rifampin in chronic plaque psoriasis. British Journal of Medicine and Medical Research.
2014;
4
(17)
:
3248-54
.
-
Talbott
W.,
Duffy
N..
Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. American Journal of Clinical Dermatology.
2015;
16
:
147-65
.
-
Tang
T. Y.,
Li
F.,
Affleck
A.,
Donaldson
J. H.,
Chouliara
Z..
Current evidence on the effectiveness of systemic herbal medicine for psoriasis: A systematic review with meta-analysis. Global Dermatology.
2015;
2
(3)
:
117-27
.
-
Kalaaji
A. N.,
Wahner-Roedler
D. L.,
Sood
A.,
Chon
T. Y.,
Loehrer
L. L.,
Cha
S. S..
Use of complementary and alternative medicine by patients seen at the dermatology department of a tertiary care center. Complementary Therapies in Clinical Practice.
2012;
18
:
49-53
.
-
Grassberger
M.,
Hoch
W..
Ichthyotherapy as alternative treatment for patients with psoriasis: a pilot study. Evidence-Based Complementary and Alternative Medicine.
2006;
3
:
483-8
.
-
Matz
H.,
Orion
E.,
Wolf
R..
Balneotherapy in dermatology. Dermatologic Therapy.
2003;
16
:
132-40
.
-
Wang
L.,
Yang
H.,
Li
N.,
Wang
W.,
Bai
Y..
Acupuncture for psoriasis: protocol for a systematic review. BMJ Open.
2015;
5
:
e007526
.
-
Rezaeizadeh
H.,
Alizadeh
M.,
Naseri
M.,
Ardakani
M. S..
The traditional iranian medicine point of view on health and.disease. Iranian. Journal of Public Health.
2009;
38
:
169-72
.
-
Mehrbani
M.,
Choopani
R.,
Fekri
A.,
Mehrabani
M.,
Mosaddegh
M.,
Mehrabani
M..
The efficacy of whey associated with dodder seed extract on moderate-to-severe atopic dermatitis in adults: A randomized, double-blind, placebo-controlled clinical trial. Journal of Ethnopharmacology.
2015;
172
:
325-32
.
-
Shaik
Y. B.,
Castellani
M. L.,
Perrella
A..
Role of quercetin (a natural herbal compound) in allergy and inflammation. Alternative Medicine Review.
2008;
13
:
66-7
.
-
Patel
S.,
Sharma
V.,
Chauhan
N. S.,
Dixit
V. K..
An updated review on the parasitic herb of Cuscuta reflexa Roxb. Journal of Chinese Integrative Medicine.
2012;
10
:
249-55
.
-
Bonifati
C.,
Berardesca
E..
Clinical outcome measures of psoriasis. Reumatismo. 2007; 59(1s): 64-7. Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLoS One.
2012;
7
:
e52935
.
-
Armstrong
April W,
Schupp
Clayton,
Wu
Julie,
Bebo
Bruce.
Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS one.
2012;
7
(12)
:
e52935
.
-
Langan
S. M.,
Seminara
N. M.,
Shin
D. B.,
Troxel
A. B.,
Kimmel
S. E.,
Mehta
N. N..
Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. The Journal of Investigative Dermatology.
2012;
132
:
556-62
.
-
Finlay
A. Y..
Quality-of-Life Issues and Economic Burden of Psoriasis and Atopic Dermatitis. Clinical Drug Investigation.
1995;
10
:
1-6
.
-
Reich
A.,
Heisig
M.,
Phan
N. Q.,
Taneda
K.,
Takamori
K.,
Takeuchi
S..
Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Dermato-Venereologica.
2012;
92
:
497-501
.
-
Services
US Department of Health and Human.
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. National Institutes of Health, National Cancer Institute.
2009;
4
(3)
:
1-94
.
-
Poulin
Y.,
Bissonnette
R.,
Juneau
C.,
Cantin
K.,
Drouin
R.,
Poubelle
P. E..
XP-828L in the treatment of mild to moderate psoriasis: randomized, double-blind, placebo-controlled study. Alternative Medicine Review.
2007;
12
:
352-9
.
-
Albanesi
C.,
De Pità
O.,
Girolomoni
G..
Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clinics in Dermatology.
2007;
25
:
581-8
.
-
Coimbra
S.,
Figueiredo
A.,
Castro
E.,
Rocha-Pereira
P.,
Santos-Silva
A..
The roles of cells and cytokines in the pathogenesis of psoriasis. International Journal of Dermatology.
2012;
51
:
389-95
.
-
Srinagar
J.,
Hassan
I..
Evaluation of the antioxidant defense status in psoriasis. Iranian Journal of Dermatology.
2014;
17
(4)
:
117-21
.
-
May
B. H.,
Zhang
A. L.,
Zhou
W.,
Lu
C. J.,
Deng
S.,
Xue
C. C..
Oral herbal medicines for psoriasis: a review of clinical studies. Chinese Journal of Integrative Medicine.
2012;
18
:
172-8
.
-
Drouin
R.,
Moroni
O.,
Cantin
K.,
Juneau
C..
A double-blind, placebo-controlled, randomized trial of XP-828L (800 mg) on the quality of life and clinical symptoms of patients with mild-to-moderate psoriasis. Alternative Medicine Review.
2008;
13
:
145-52
.
-
Prussick
R.,
Prussick
L.,
Gutman
J..
Psoriasis Improvement in patients using glutathione-enhancing, nondenatured whey protein isolate: A Pilot Study. The Journal of Clinical and Aesthetic Dermatology.
2013;
6
:
23-6
.
-
Sharma
R.,
Shah
N..
Health benefits of whey proteins. Nutrafoods.
2010;
9
(4)
:
39-45
.
-
Patel
S..
Functional food relevance of whey protein: A review of recent findings and scopes ahead. Journal of Functional Foods.
2015;
19
:
308-19
.
-
Markus
C. R.,
Olivier
B.,
de Haan
E. H..
Whey protein rich in α-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. The American Journal of Clinical Nutrition.
2002;
75
:
1051-6
.
-
Bai
M.,
Liu
H.,
Xu
K.,
Oso
A. O.,
Wu
X.,
Liu
G..
A review of the immunomodulatory role of dietary tryptophan in livestock and poultry. Amino Acids.
2017;
49
:
67-74
.
-
Yu
E. S.,
Min
H. J.,
An
S. Y.,
Won
H. Y.,
Hong
J. H.,
Hwang
E. S..
Regulatory mechanisms of IL-2 and IFNgamma suppression by quercetin in T helper cells. Biochemical Pharmacology.
2008;
76
:
70-8
.
-
Phan
T. T.,
See
P.,
Tran
E.,
Nguyen
T. T.,
Chan
S. Y.,
Lee
S. T..
Suppression of insulin-like growth factor signalling pathway and collagen expression in keloid-derived fibroblasts by quercetin: its therapeutic potential use in the treatment and/or prevention of keloids. British Journal of Dermatology.
2003;
148
:
544-52
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 8 (2018)
Page No.: 2620-2632
Published on: 2018-08-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7050 times
- Download PDF downloaded - 2372 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress




