Antimicrobial resistance in uropathogen isolates from patients with urinary tract infections
Abstract
Aims: Because of uncontrolled and widespread use of antibiotics, the resistance pattern of uropathogens is changing drastically, specifically in developing countries, such as Bangladesh. The aim of the study was to identify the common Urinary Tract Infection (UTI) causing pathogens in the city of Jessore, Bangladesh and to check the performance of available antibiotics used by those patients.
Study Design: Random 100 UTI patients who exhibited general UTI symptoms were included in our cross-sectional study. A medical proforma was prepared to input the information associated with the experiment including symptoms of patient’s age, sex, laboratory diagnosis and antimicrobial susceptibility.
Place and Duration of Study: Department of Microbiology, University of Science and Technology, Jessore 7408 and Pharmacy Discipline, Life Science School, Khulna University, Khulna 9208, Bangladesh, between June 2013 and July 2014.
Methodology: Urine samples from 100 suspicious urinary tract infected patients were collected as described by Thomson and Miller. Bacterial isolates were tested to identify the bacterial species and to evaluate their antimicrobial susceptibility by Kirby-Bauer disk diffusion technique against some common antibiotics. Epidata® computer program 3.1 and SPSS version 16 statistical software used for confidence interval (CI) and P value, which were defined as P value is <0.05 and CI was set at 95% level of significance for all the proportions.
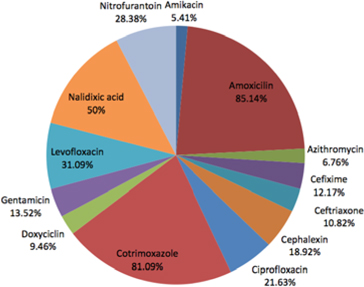
Results: Among 100 samples, 74 samples showed positive for cultures adversely responsible for UTIs. About 5 different species of uropathogens were identified from 74 cases. Comparative prevalence of E. coliwas detected in 69 of the 100 samples (69%), while Staphylococcus spp was found in 18 samples (18%), Pseudomonas aeruginosa in 8 samples (8%), and Klebsiella pneumoniae in 6 samples (6%), respectively. Comparative antibiotic resistance profile showed that most of the strains were highly resistant to Amoxicillin (85.14%) and Cotrimoxazole (81.08%). On the contrary, the strains showed significant sensitivity to Amikacin (94.59%), Azithromycin (93.24%), Doxycycline (90.54%), and Ceftriaxone (89.18%), respectively showed significant sensitivity.
Conclusion: Our results could be helpful to compel rational antibiotic use for UTI. High resistance of uropathogens to antibiotics, such as Amoxicillin and Cotrimoxaxole, has been observed in a significant number of patients in the developing world, such as Bangladesh. Our studies may provoke further investigations into the mechanisms of antibiotic resistance of particular microbes.
Introduction
In developing countries, UTI is a common experience in clinical practice. Each year up to 150 million individuals are affected by UTIs Stamm and Norrby, 2001. Colonization of normal and opportunistic microflora is a main causal agent for UTIs. In the third world, causative factors for UTI include poor hygiene, long time catheterization, uncontrolled sexual intercourse, pregnancy and spermicidal contraception Manges et al., 2008.
However, the causal organisms are easily predictable and UTI can be classified by different criteria. The most predominant for UTIs are gram-negative bacteria, particularly Escherichia coli, which is mainly responsible for the high prevalence Moges et al., 2002.
In those with frequent infections, low-dose antibiotics may be taken as a preventative measure. For recurrent and complicated infections, intravenous daily antibiotics or prolonged antibiotics course may be effective. Unfortunately, several studies have reported that many uropathogens became resistant to a wide range of antibiotics due to abuse, over dosage use, nonprescribed use, uncompleted dosages, and ease of access to antimicrobial drugs Tambekar et al., 2008.
In the third world, considering resource constraints, it is a matter of regret that routine analysis of antibiotic resistance is impractical. Resistance of uropathogens to antibiotics is now a global concern Gupta, 2001Karlowsky et al., 2002Kurutepe et al., 2005. Variations in resistance pattern of different antibiotics are known to occur in different geographical area as well as in the same country Schito et al., 2009. Around the Indian subcontinent, including Bangladesh, there is a lack of data on uropathogens which develop antibiotic resistance. Given this background, the aim of the study was to identify the common UTI causing pathogens in urine samples of a local community of Bangladesh and to check the performance of available antibiotics used by those patients. Our results could be helpful to compel rational antibiotic use for UTI and to provoke studies into the mechanisms of resistance of those particular microbes.
Material and methods
Study design
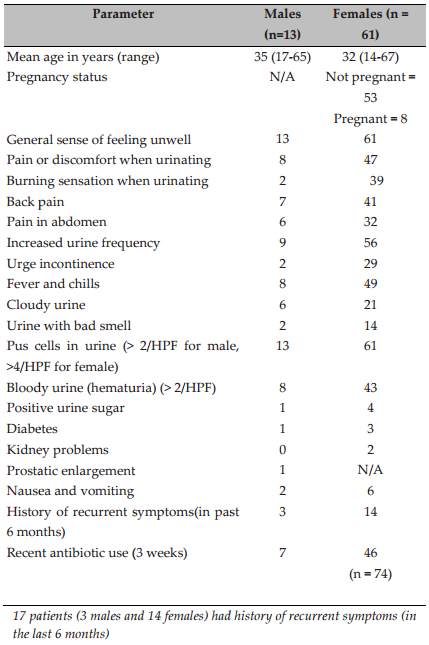
A random group of 100 symptomatic UTI patients were included in our cross-sectional study; these patients exhibited suprapubic pain, back pain, burning sensation, discomfort during urination, urinary frequency, urge incontinence and fever. UTI can be classified by different criteria; it can be symptomatic or asymptomatic, and complicated or uncomplicated. Uncomplicated UTI is infection associated with kidney or bladder without causing any functional abnormality, whereas complicated UTI is associated with diabetes mellitus, kidney transplantation, pregnancy, prostate enlargement, metabolic or immunologic illness. Infection is usually considered complicated when structural and functional abnormalities in the urinary tract and associated parts are found; otherwise, it is considered uncomplicated. In this study UTI patients were not investigated to find any structural and functional difficulties. Therefore, it was difficult to designate whether their UTIs were complicated or uncomplicated. However, 17 patients (3 males and 14 females) had history of recurrent symptoms for the last 6 months. Recurrent infections are typical with complicated UTIs so it is likely that these 17 patients would be classified as complicated UTIs.
A medical proformawas prepared to input the information associated with the study, including symptoms of the patients, their age, sex, laboratory diagnosis, and antimicrobial susceptibility. Bacterial isolates from suspected patients were tested to identify the species and the samples were evaluated for antimicrobial susceptibility by Kirby-Bauer disk diffusion techniques Bauer et al., 1966.
Urine sample collection and determination of infection
Urine samples were collected aseptically at the midstream level of urination with sterile labeled containers (100 ml) as described by Thomson and Miller Thomson, 2003. To define ‘positive UTI’ microscopic enumeration of urine leucocytes (WBCs) and quantitative urine culture were measured. In addition, enumeration of red blood cells (RBCs) was also determined to support ‘positive UTI’. For microscopic enumeration, 10-15 ml of mid-stream urine sample was centrifuged at 1500 to 3000 rpm for 5 min.
Supernatant was decanted using pipette, leaving 1 ml urine at the bottom of the test tube. Sediment in bottom of the test tube was resuspended with the retaining 1 ml of urine. Then 15 μl of resuspended sediment was applied on aaglass slide using calibrated pipette.
Cover slip was used to avoid any possible contamination. Then the slide was examined with a light microscope using the high dry objective (40x). At least 3 and 5leucocytes per high power field (HPF) in males and females, respectively, were considered as positive UTI.
A finding of 3 RBCs/HPF in both sexes was considered as a supportive indication for positive UTI. All positive samples were further subjected to urine culture. For each patient 10μl urine sample was plated for quantitative culture on nutrient agar media prepared according to the established methods. A cutoff point of 105 colony forming a unit (CFU)/ml was considered as positive culture Cheesbrough, 2006.
Isolation and identification of uropathogens
10 μl urine samples plated on 20 ml of 3.62% cysteinelactose-electrolyte-deficient (CLED) media (Oxoid UK) was poured into a Petri dish. After drying, the plates were incubated at 350C (±1) for 24 h. Then, colonies were identified by the individual characteristics of the various organisms by colony size, colony shape, and colony color. After observing the bacterial colony and growth characteristics, a series of biochemical tests, including methyl red test, sugar fermentation, catalase, coagulase, urease, citrate utilization, and indole production. were conducted according to the standard methods Kass et al., 2000.
Antibiotic susceptibility test
Antibiotic susceptibility test was performed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI)CLSI, 2009. Muller Hinton II agarwas used in an antibiotic susceptibility test by Kirby-Bauer disk diffusion techniques Bauer et al.,1966. The Muller Hinton II agar media was prepared according to the manufacturer’s recommendation (38g/l). Suspected organisms were grown on peptone water and allowed to incubate at 37°C for 6 to 8h. Peptone water was swabbed on Muller-Hinton agar petri dishes and dried for 8 to 10 min. After that, commercially produced antibiotic discs of different potency were placed into the media by sterile forceps and refrigerated for 3 to 4h. The zones of inhibition for different antibiotics were determined after 18 to 24h of incubation. The following antibiotic discs were used: Amikacin (30μg), Amoxicillin (10μg), Azithromycin (15μg), Cefixime (5μg), Ceftriaxone (30μg), Cephalexin (30μg), Ciprofloxacin (5μg), Cotrimoxazole (25μg), Doxycycline (30μg), Gentamicin (10μg), Levofloxacin (5μg), Nalidixic acid (30μg), and Nitrofurantoin (50μg).The diameter of inhibition zone was measured by digital slide calipers and interpreted as ‘sensitive’, ‘intermediate’ or ‘resistant’, according to CLSI standard interpretative charts.
Data collection and statistical analysis
All the data were routinely collected and were entered twice into the Epidata ® computer program 3.1 (http://www.epidata.dk)Lauritsen, 2008. Data were then validated and analyzed by simple percentages among related variables. The data were analyzed using SPSS version 16 statistical software for confidence interval (CI) and Pvalue. Statistical significance was defined when P value is <0.05. The confidence interval was set at 95% level of significance for all the proportions.
Results and discussion
Demographic and clinical characteristics of patients
Out of the 100 patients tested, only 74 exhibited positive UTI, based on microscopic enumeration of leukocytes and RBCs, and quantitative urine culture, as described in Methods. Prevalence of UTI was found to be highest in the ages between 31-40 years, and females were mostly affected ( Figure 1 ).
It has been reportedthat in women between the ages of 20-40 years, 25%-35% have had a UTI (http://www.epidata.dk)which merges with the results of our study. Interestingly, from a study of Meerut City, India it was found that the prevalence of UTI is significantly higher in females than in males (females: 73.57%, age group of 26–36 years) Foxman et al., 2000. In case of males, elderly males of ≥48 years showed higher prevalence of UTI. In this study, we found higher prevalence of UTI in the age group between 26-36 years. Details of demographic and clinical characteristics of the patients have been mentioned in Table 1 .
Several explanations for higher UTI prevalence in females are shorter urethra and its anatomical relation to the genitourinary tract for women. This may cause pushing of bacteria into the urethra during intercourse and sometimes massaging up of bacteria from the urethra into the bladder during pregnancy/child birth Prakash and Saxena, 2013. The particular age groups of 31-40 years are more hazard-prone to UTI. It should be pointed out that the patients were selected randomly and more female patients were available with UTI symptoms which could impact our findings.Fifty three participants (71.62%) reported antibiotic use prior to participating in this study. Seventeen participants (22.97%) had history of recurrent symptoms in past the 6 months. Statistically, there was highly significant difference between males and females according to the distribution of the isolated bacteria (P value ≤ 0.05).
Antibiotic susceptibility and resistance of the uropathogens
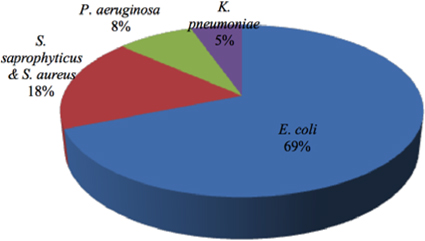
Out of 74 UTI patients, the prevalence of E. coli was 51(69%), whereas forStaphylococcusspp. the number was only 13(18%). Eight were identified as S. saprophytics while 5 were identified as S. aureus. On the other hand, the prevalence ofP. aeruginosa (n = 6) and K. pneumonia (n = 4) were considerably low (8% and 5%, respectively) ( Figure 2 ). E. coli was the most predominant uropathogenfound in our study, which is supported by everal previous works (http://dx.doi.org/10.1155/2013/749629) Okonko et al., 2009. For example, a study from Kathmandu, everal previous works (http://dx.doi.org/10.1155/2013/749629) Okonko et al., 2009. For example, a study from Kathmandu, Nepal showed that E. coli was the most prevailing organism ganism (81.3%) Kamenski et al., 2012. E. coli was also prevalent in studies from Tumkur and Bangalore, India (59.2%) Shanthi and Kayathri, 2012.
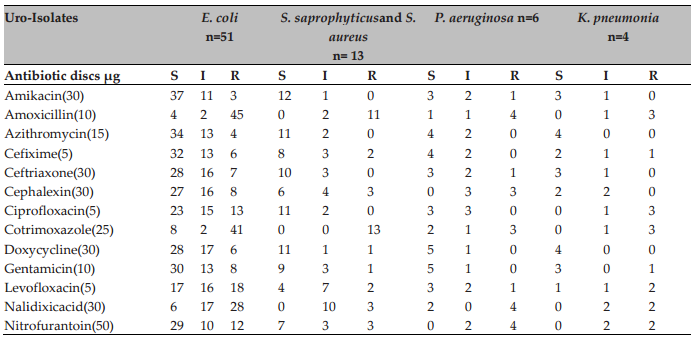
During analysis of antibiotic susceptibility most of the organisms were moderately resistant to Amoxicillin, Cotrimoxazole, and Nalidixic acid. E. coli showed 94.12% susceptibility to Amikacin. Staphylococcusspp. (S. saprophyticusand S. aureus) was susceptible to Amikacin (13/13, 100%) and resistant to Cotrimoxazole (0/13, 0%). P.aeruginosa was found to show susceptibility to Azithromycin, Cefixime, Ciprofloxacin, Doxycycline and Gentamicin (6/6, 100%) and 66.66% resistant to Amoxicillin, Nalidixic Acid, Nitrofurantoin (4/6,66.66%). K. pneumoniaewas found to be susceptible to Amikacin, Azithromycin, Ceftriaxone, Doxycycline (4/4, 100%) and 75% resistance to Amoxicillin, Ciprofloxacin, Cotrimoxazole (3/4, 75%) ( Table 2 ). Table 2 also showed that no (0/4, 0%) K. pneumoniastrains were resistant to Cephalexin and 2 (2/4, 50%) strains were resistant to Levofloxacin.
UTI in adults, gynaecological surgery in females, obstructive uropathy in males and complicated UTI in females with the occurrence of UTI with Ciprofloxacin resistant E. coli were noted Baral et al., 2012. Another study from India revealed that Ampicillin has the highest resistance by different uropathogens i.e. 80.4% followed by Ciprofloxacin (73%), Amoxicillin (70.4%), Norfloxacin (53.3%) while Penicillin, Furomycin, Azithromycin, Doxycycline, Linezolid and Novobiocin offered least resistant Shanthi and Kayathri, 2012.
Comparative prevalence of bacterial species in the urine samples.
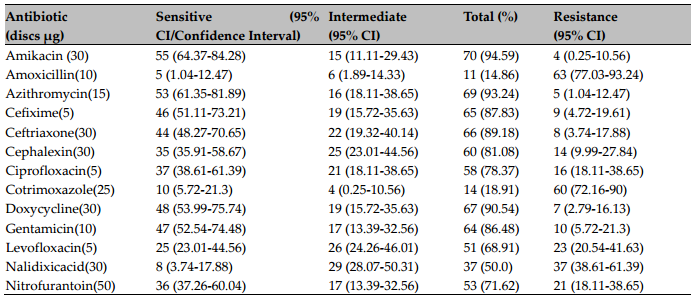
However, our findings indicate that Amikacin is the most effective antimicrobial agent. Mos and co- workers also showed high sensitivity of bacteria to Amikacin (75%)(http://www.biomedcentral.com/1756- 0500/5/38). In Uganda and other developing countries, high level of resistance to the most commercially available antibiotics were found in UTI patients (Majumdar, 2012; Mandal et al., 2012). For Nitrofurantoin and Nalidixic Acid, P. aeruginosa and K. pneumonia showed considerable insensitivity. A list of the relative proportions of susceptibility and resistance profiles from all uropathogens against each antibiotic is presented in Table 3 , along with their confidence intervals set at 95% level of significance.
After the combination of sensitive and intermediate readings, the most favorable profiles were obtained with Amikacin (94.59%), Azithromycin (93.24%), Dox- ycycline (90.54%) and Ceftriaxone (89.18%). Uropathogen data showed that the uropathogens showed lowest (5.41%) resistance to Amikan, and highest resistance to Amoxicillin (85.14%)( Figure 3 ).
Some factors which largely contribute to the evolution of microbial drug resistance are incomplete doses (to cut treatment cost), access to antibiotics without prescription, prescription of higher generation antimicrobials, over prescription, and use of antibiotics without laboratory diagnosis Majumdar, 2012Mandal et al., 2012. It has been reported that antimicrobials used in agriculture, farm, household and industry are sources of antimicrobial resistance as well Mwaka et al., 2012.
Conclusion
In our study, resistance to Amoxicillin, Cotrimoxazole,and Nalidixic acid were found in alarming rates. Somehow these drugs have lost their capability to inhibit uropathogens. In addition, Levofloxacin, Cephalexin and Ceftriaxone show trends of resistance.
Azithromycin, Amikacin, and Cefixime are relatively satisfactory and effective in treating UTIs. Our research has been confined to a particular region of Bangladesh. Larger samples sizes from other regions of Bangladesh can lead to more significant results. Therefore, it is increasingly important to study these resistant uropathogens, including mechanisms of resistance, in order to develop effective future drugs.
Ethical considerations
The present study was carried out in accordance with The Declaration of Helsinki and was approved by the Institutional Review Board of the Department of Microbiology, Jessore University of Science and Technology (reference no. JUST/DM/09/12/12). Written consent was obtained from each patient of the study. The investigators ensured the protection of health, dignity, integrity, right to self‐determination, privacy, and confidentiality of personal information of participants.
References
-
P.
Baral,
S.
Neupane,
B.
Marasini,
K.
Ghimire,
B.
Lekhak,
B.
Shrestha.
High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Research Notes.
2012;
5
:
38
.
-
A.W.
Bauer,
W.M.
Kirby,
J.C.
Sherris,
M.
Turck.
Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol.
1966;
45
:
493-496
.
-
M.
Cheesbrough.
Details of Part 1. In District Laboratory Practice in Tropical Countries. Cambridge University Press (CUP).
2006;
:
380-380
.
-
C.L.S.I.
CLSI.
Performance of standards for antimicrobial disk susceptibility tests; approved standards., Vol 29, 10th edn. M02-A10: Wayne, PA: CLSI..
2009
.
-
B.
Foxman,
R.
Barlow,
H.
D'Arcy,
B.
Gillespie,
J.D.
Sobel.
Urinary Tract Infection. Annals of Epidemiology.
2000;
10
:
509-515
.
-
K.
Gupta.
Increasing Antimicrobial Resistance and the Management of Uncomplicated Community-Acquired Urinary Tract Infections. Annals of Internal Medicine.
2001;
135
:
41
.
-
G.
Kamenski,
G.
Wagner,
S.
Zehetmayer,
W.
Fink,
W.
Spiegel,
K
Hoffmann.
Antibacterial resistances in uncomplicated urinary tract infections in women: ECO·SENS II data from primary health care in Austria. BMC Infect Dis.
2012;
12
:
222
.
-
J.A
Karlowsky,
L.J
Kelly,
C
Thornsberry,
M.E
Jones,
DF
Sahm.
Trends in Antimicrobial Resistance among Urinary Tract Infection Isolates of Escherichia coli from Female Outpatients in the United States. Antimicrob Agents Chemotherapy.
2002;
46
:
2540-2545
.
-
E.J.
Kass,
K.M.
Kernen,
J.M.
Carey.
Paediatric urinary tract infection and the necessity of complete urological imaging. BJU International.
2000;
86
:
94-96
.
-
S.
Kurutepe,
S.
Surucuoglu,
C.
Sezgin,
H.
Gazi,
M.
Gulay,
B.
Ozbakkaloglu.
Increasing antimicrobial resistance in Escherichia coli isolates from community-acquired urinary tract infections during 1998-2003 in Manisa, Turkey. Jpn J Infect Dis.
2005;
58
:
159-161
.
-
Lauritsen.
EpiData Data Entry, Data Management and basic Statistical ASystem. OdenseD: EpiDa-ta Association.
2008
.
-
S.
Majumdar,
Singh.
Resistant Escherichia coli and Klebsiella Spp. in Community-Acquired Urinary Tract Infections in Rural Kanpur. India J Clin Diagnos.
2012;
6
:
978-981
.
-
J.
Mandal,
N.S.
Acharya,
D
Buddhapriya,
SC
Parija.
Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin resistant Escherichia coli. The Indian journal of medical research.
2012;
136
:
842
.
-
A.R.
Manges,
H
Tabor,
P
Tellis,
C
Vincent,
P-P
Tellier.
Endemic and Epidemic Lineages of Escherichia coli that Cause Urinary Tract Infections. Emerg Infect Dis.
2008;
14
:
1575-1583
.
-
A.F.
Moges,
A.
Genetu,
G.
Mengistu.
Antibiotic sensitivities of common bacterial pathogens in urinary tract infections at Gondar Hospital, Ethiopia. East African Medical Journal.
2002;
79
.
-
A.
Mwaka,
H.
Mayanja-Kizza,
E.
Kigonya,
D.
Kaddu-Mulindwa.
Bacteriuria among adult non-pregnant women attending Mulago hospital assessment centre in Uganda. African health sciences.
2012;
11
.
-
I.
Okonko,
L.
Ijandipe,
O.
Ilusanya,
O.
Donbraye-Emmanuel,
J.
Ejembi,
A.
Udeze,
O.
Egun,
A.
Fowotade,
A.
Nkang.
Incidence of urinary tract infection (UTI) among pregnant women in Ibadan, South- Western Nigeria. African Journal of Biotechnology.
2009;
8
.
-
Saxena RS
Prakash D.
Distribution and Antimicrobial Susceptibility Pattern of Bacterial Pathogens Causing Urinary Tract Infection in Urban Community of Meerut City, India. ISRN Microbiology.
2013;
2013
:
1-13
.
-
G.C.
Schito,
K.G.
Naber,
H.
Botto,
J.
Palou,
T.
Mazzei,
L.
Gualco,
A.
Marchese.
The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. International Journal of Antimicrobial Agents.
2009;
34
:
407-413
.
-
J.
Shanthi,
S.
Kayathri.
Incidence, distribution and antibiogram of uropathogens isolated from patients with urinary tract infections. Adv Applied Sci Res.
2012;
3
:
3410-3414
.
-
Walter E.
Stamm,
S.R.
Norrby.
Urinary Tract Infections: Disease Panorama and Challenges. The Journal of Infectious Diseases.
2001;
183
:
S1-S4
.
-
D.H.
Tambekar,
D.V.
Dhanorkar,
S.R.
Gulhane,
M.N.
Dudhane.
Prevalence And Antimicrobial Susceptibility Pattern Of Methicillin Resistant Staphylococcus aureus From Healthcare And Community Associated Sources. African Journal of Infectious Diseases.
2008;
1
.
-
M.
Thomson.
Specimen collection, transport, and processing: bacteriology. In In Manual of Clinical Microbiology, B.E. Edited by Murray PR, Jorgensen JH, Pfaller MA, Yolken RH, ed.. Washington DC: American Society of Microbiology.
2003
.
Comments
Downloads
Article Details
Volume & Issue : Vol 2 No 05 (2015)
Page No.: 263-269
Published on: 2015-05-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7760 times
- Download PDF downloaded - 2181 times
- View Article downloaded - 10 times
 Biomedpress
Biomedpress