Abstract
Introduction: Jaundice is the most common condition requiring medical attention in newborns. Phototherapy is the standard treatment for jaundice. However, in recent decades, phototherapy has been considered as an oxidative stress that can cause lipid peroxidation and damage to DNA. Accordingly, the present study was conducted to examine the possible effects of phototherapy on the Pro-oxidant/Antioxidant Balance (PAB) in jaundiced newborns.
Methods: The present clinical trial was conducted on 70 icteric term neonates admitted to Ghaem Hospital of Mashhad, Iran between February 2013 and February 2015. The study population consisted of all term neonates who were admitted to the hospital within 2 to 14 days after birth for unconjugated hyperbilirubinemia (bilirubin levels > 15 mg/dl) and were treated with phototherapy. Neonates’ and mothers’ characteristics, along with the cause of jaundice, were collected through a researcher-made questionnaire, and neonates’ bilirubin levels and PAB values were simultaneously checked before, during and after the phototherapy. Finally, bilirubin levels and PAB values were compared using statistical methods.
Results: According to the results, neonates’ underlying characteristics were not statistically different. The average and standard deviation of bilirubin levels and PAB values were, respectively, 18.90±2.97 and 16.29±9.83 (before phototherapy), 15.71±3.16 and 29.63±12.56 (during phototherapy), and 12.37 ±3.57 and 40.91 ±13.35 (after phototherapy).
Conclusion: The results of this study demonstrate that decreased levels of bilirubin after phototherapy cause a shift in the PAB value in favor of oxidants.
Background
Jaundice is the most common condition requiring medical attention in newborns, and phototherapy is the standard treatment for that 1. Phototherapy changes unconjugated bilirubin into oxidized bilirubin and its structural isomers that can be easily excreted in the stool and urine 2. Phototherapy is known as a safe method that is well-tolerated by neonates 3. Over the past decade, however, phototherapy has been considered as an oxidative stress that can cause lipid peroxidation and damage to cellular DNA 4567.
Oxidative stress can be defined as an imbalance between the amounts of Reactive Oxygen Species (ROS) and extracellular antioxidant defense systems8. Erythrocytes are extremely sensitive to lipid peroxides because their membranes are rich in unsaturated fats, and they are responsible for supplying oxygen and transition metal catalysts. Neonates’ erythrocyte membranes are sensitive to oxidative damage above their pro-oxidant capacity 9. Bilirubin is a unit stimulating the production of oxygen and acting as an antioxidant, especially when combined with albumin. Therefore, the bilirubin-albumin ratio is important in determining the type of activity 1011.
Traditional phototherapy has negative impacts on the antioxidant defense system in term neonates as neonates have limited protective antioxidant capacity 6. This limited capacity is even more serious in preterm neonates and those with low birth weight. Oxidative damage plays an important role in the pathogenesis of many neonatal diseases 1213.
Several studies have emphasized the role of bilirubin as an antioxidant; accordingly, it seems that the antioxidant capacity of plasma bilirubin is higher compared to urates, alpha-tocopherol or even ascorbates 14. Trapping free radicals or toxic products of oxygen done by bilirubin has been mentioned in previous studies. Unconjugated bilirubin can effectively eliminate monovalent oxygen and make it react with superoxide anions and peroxyl radicals 15. In an in vitro study, it was shown that florescent light decreases the activity of ATPase and increases the rate of lipid peroxidation 16.
A critical intracellular balance exists between the formation of free radicals, antioxidant defense and regenerative systems; this means that in an appropriate physiological condition, there is a balance between antioxidants and pro-oxidants, and that free radicals are neutralized by the antioxidant system 17. Stressful conditions stimulate the production of free radicals by reducing the coupling rate of oxidation and phosphorylation in the mitochondria, leading to increased electron leakage and overproduced superoxide radicals. When the production of free radicals exceeds the capacity of the antioxidant capacity system for neutralizing, lipid peroxidation causes damage to unsaturated lipids in cells membranes, amino acids in proteins, and nucleotides in DNA, resulting in the disintegration of cells and membranes. This situation is exacerbated by reduced efficiency of the immune system and adverse changes in the cardiovascular, nervous and muscle systems, and the brain, due to increased rates of lipid peroxidation. Thus, many symptoms and complications may occur following a disturbance in the Pro-oxidant/Antioxidant Balance (PAB) 1718.
Akisü et al. conducted a study on 36 jaundiced neonates and found no significant difference in serum levels of vitamin E and red blood cell antioxidant enzyme activities (e.g. of superoxide dismutase, catalase, and glutathione peroxidase) between the experimental and control groups before and 72 hours after phototherapy. The results of Akisü’s study showed no damage to some components of the antioxidant system after the phototherapy; however, the overall PAB value was not determined in that study. Accordingly, possible positive or negative effects of phototherapy on the antioxidant system were not determined with certainty. Therefore, the present study was conducted to examine the possible effects of phototherapy on the PAB 19.
Methods
The present clinical trial was conducted on icteric term neonates admitted to either the Neonatal Intensive Care Unit (NICU) or the Pediatric Emergency Unit of Ghaem Hospital of Mashhad, Iran between February 2013 and February 2015. Samples were selected based on the purposive sampling method. In this study, 89 neonates were initially examined, but 19 neonates were excluded from the study due to hemolysis and inability to assess their PAB values.
The study population consisted of all term neonates who were admitted to the hospital within 2 to 14 days after birth for idiopathic unconjugated hyperbilirubinemia (bilirubin levels > 15 mg/dl) and were treated with phototherapy. The present project was approved by the Ethics Committee of Mashhad University of Medical Sciences (Code 922433). Parental consent forms were collected before the initiation of the study. No samples were taken from the examined neonates and only the remaining serum of their bilirubin tests was used. The exclusion criteria included severe birth defects, eclampsia/preeclampsia in mothers, birth asphyxia, respiratory distress, sepsis, hemolytic hyperbilirubinemia, Rh or ABO incompatibility, a positive direct Coombs test result, and signs of jaundice during the first 24 hours after birth.
The neonates’ information, including age of onset of jaundice, gender, birth weight, gestational age and Apgar score, were collected, as were the mothers’ information, which included age, parity, pregnancy-related problems, mode of delivery, history of other children’s hospitalizations, blood transfusions, and blood type. Additionally, neonatal bilirubin level, hematocrit level, direct and indirect Coombs tests, reticulocytes, Glucose-6-phosphate dehydrogenase (G6PD), Thyroid Stimulating Hormone (TSH), T4, and PAB were measured and recorded. Phototherapy was performed based on principles defined by the American Academy of Pediatrics (AAP); it was only interrupted by breastfeeding, changing of diapers, and taking blood samples. Age of onset of phototherapy as well as Serum Total Bilirubin (STB) levels, at the beginning and end of the phototherapy process, were also recorded. For each neonate, at least 0.2 cc of blood was separated from the neonate’s total blood sample that was sent to the laboratory. The separated blood samples were transported to Bu-Ali Research Institute for the measurement of PAB values.
The solutions were prepared as indicated. The standard solutions were made by mixing varying proportions (0-100%) of 250 μM hydrogen peroxide with 3 mM uric acid in 10 mM NaOH. In order to prepare the TMB cation, 60 mg of TMP powder was dissolved and mixed well at a ratio of 10-20 ml of the solution. Then, the prepared solution was placed in the dark for 2 hours. Next, 25 units of peroxidase enzyme were added to 20 ml of the solution, and placed at 20oC. For preparation of the TMB solution, 200 ml of TMB was added into 10 ml of acetate buffer (0.05 M buffer, pH 5.8); the working solution was prepared by mixing 1 ml of TMB cation with 10 ml of TMB solution. The prepared solution was placed in a dark, dry place for 2 minutes. Next, 10 μL of each sample was mixed with 200 μL of the working solution and placed in a 96-well plate in the dark at 37oC for 12 min. At the end of the process, 100 μL of 2N Hall was added to each well and measured in an ELISA plate reader at 450 nm and 620 nm wavelengths.
A standard curve showing PAB values in HK units was created based on the standard samples. In that curve, the percentage of hydrogen peroxide in the standard solution was shown. PAB values were calculated and shown on the curve.
Using the SPSS-16 software, the collected data were analyzed. Mean, standard deviation (SD), and frequency tables/charts were used to describe the data. Student’s t-test, chi-square test, and general linear modeling were used to analyze the data.
Results
The results of the statistical tests showed that the underlying characteristics were not significantly different before and after phototherapy Table 1.
| Group | Intervention | Result | |
| Variable Index | Mean | SD | |
| Age on admission (day) | 7.4318 | 4.66041 | P=0.068 |
| Weight on admission (gr) | 3133.5211 | 560.63828 | P=0.895 |
| Birth weight (gr) | 3144.0132 | 605.64897 | P=0.995 |
| Maternal age (year) | 29.5333 | 5.70598 | P=0.616 |
| Baseline hematocrit level (%) | 44.90 | 6.96288 | P=0.592 |
| Baseline bilirubin level | 18.90 | 2.97369 | P= 0.182 |
| Secondary bilirubin level | 15.7184 | 3.16658 | P= 0.512 |
| Time interval between baseline and secondary bilirubin test (hour) | 8.3103 | 4.34905 | P= 0.001 |
| Tertiary bilirubin level | 13.3508 | 3.72060 | P= 0.633 |
| Initial PAB value | 16.2920 | 9.83746 | P= 0.417 |
| Secondary PAB value | 29.6327 | 12.56828 | P= 0.605 |
| Tertiary PAB value | 40.9163 | 13.35465 | P=0.663 |
| Baseline conjugated bilirubin level | 0.4220 | 0.21718 | P=0.001 |
| Urea level | 29.8636 | 21.34173 | P= 0.594 |
| Creatinine level | 0.4605 | 0.19166 | P=0.594 |
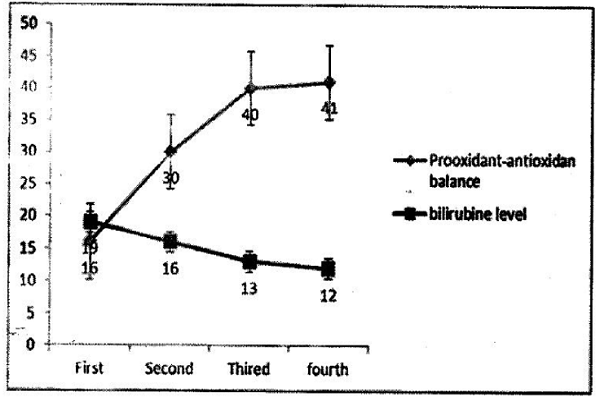
Table 2 shows the values of the underlying qualitative variables and characteristics in the neonates. Most of the infants were boys (51.7%), term (74.2%), and born naturally (42.7%) Table 2. The neonates’ bilirubin levels and PAB values are presented in Figure 1.
The mean and SD of baseline and secondary bilirubin levels were, respectively, 18.9067±2.97369 and 15.7184±3.16658, and the mean and SD of tertiary bilirubin level were, respectively, 13.3508±3.72060. The mean and SD of baseline (before phototherapy) and secondary PAB values were, respectively, 16.29±9.83 and 29.63±12.56, and the mean and SD of tertiary PAB values (after phototherapy) were, respectively, 40.91±13.35.
| Variable | Frequency (%) | |
| Gender | Male | 46 (51.7) |
| Female | 43 (48.3) | |
| Maternal age (year) | Term | 66 (74.2) |
| Preterm | 10 (11.2) | |
| Postterm | 2 (2.2) | |
| Mode of delivery | Normal Vaginal Delivery | 38 (42.7) |
| C-section | 33 (37.1) | |
| Unspecified | 18 (20.2) |
Along with phototherapy, the neonates’ bilirubin levels were reduced. The consequent changes in the neonates’ PAB values are shown in Figure 2.
Discussion
The results of this study indicated a disturbance in PAB as a result of reduced levels of bilirubin. Previously, Gopinathan and colleagues examined 31 preterm and 16 term neonates to investigate the relationship between bilirubin level and ascorbate antioxidant activity in neonatal plasma; they reported similar results to the results of the present study 20.
Akisü conducted a study on 20 term and 16 icteric preterm neonates, who needed 72 hours of continuous phototherapy, to examine the antioxidant defense system in neonates undergoing phototherapy. Their study was conducted to investigate the possible incidence of oxidative stress as a result of phototherapy. To do so, the authors measured serum levels of vitamin E and red blood cell antioxidant enzymes activities (superoxide dismutase, catalase, and glutathione peroxidase) 72 hours before and after phototherapy. The results showed no significant change in serum levels of vitamin E before and 72 hours after the phototherapy. Accordingly, they did not confirm that phototherapy creates oxidative stress 19. However, it must be considered that the mentioned study’s sample size was half of the present study’s sample size, and that a limited number of antioxidants were examined in the mentioned study while the total balance of oxidant/antioxidant system was assessed in the present study.
Oxidative stress can be defined as an imbalance between the amount of ROS and the body’s ability to detoxify those operating defense systems. This imbalance leads to damage to all molecules in the cell, such as proteins, lipids and even the DNA, and interferes with the cellular signaling system. Bilirubin absorbs blue light in the range of 460-490 nm. Various tools working with different wavelengths and light intensity are now available for phototherapy 21. Phototherapy changes unconjugated bilirubin into oxidized bilirubin and its structural isomers that can be easily excreted in the stool and urine. It has been suggested that phototherapy has a negative effect on the oxidant/antioxidant defense system, leading to increased levels of oxidative stress in neonates undergoing phototherapy treatment. Phototherapy may be associated with high levels of oxidative stress, rates of lipid peroxidation and damages to DNA. Neonates have limited antioxidant capacity and, thus, oxidative destruction plays an important role in the pathogenesis of many neonatal diseases. Considering bilirubin is an antioxidant, the role of phototherapy in PAB has not been well-understood yet and previous studies have shown conflicting results 6722.
The traditional method of phototherapy was used in the present study. Demirel and colleagues compared the Total Oxidant Status (TOS) to antioxidant status in unconjugated neonatal hyperbilirubinemia before and after traditional phototherapy and LED treatment. They concluded that increased TOS occurs after traditional phototherapy, but not after LED treatment. Moreover, they reported that the Oxidative Stress Index was significantly higher in traditional phototherapy, compared to LED treatment (p<0.05) It seems that even the type of lamp used in phototherapy can affect its outcomes 22.
In the present study, the examined neonates were term and had bilirubin levels > 15 mg/dl. They had also a stable hemodynamic condition. These characteristics were similar to characteristics of samples in a study conducted by Dahiya and colleagues in India 23; however, Dani and colleagues examined preterm neonates in their study 24. Dahiya and colleagues reported a significant increase in malondialdehyde (MDA) levels among members of the experimental group after the phototherapy (p<0.001). They also found a significant increase in superoxide dismutase (SOD) level as a result of phototherapy (p<0.001). Moreover, levels of non-enzymatic antioxidants, such as glutathione (GSH), total thiols and vitamin C, were significantly lower in the experimental group at the onset and at the end of the phototherapy. The researchers also reported that serum albumin levels significantly decreased after the phototherapy (p<0.01). Finally, they concluded that phototherapy increases levels of oxidative stress and, therefore, must be applied with caution 23. Meanwhile, Dani and colleagues examined 22 preterm neonates (gestational age <36 weeks/ age <7 days) admitted to the NICU of Florence Teaching Hospital. The neonates’ total bilirubin levels were measured 24 hours before and after the phototherapy. In their study, total bilirubin level was not significantly related to total plasma hydroperoxide concentration (TH), Total Antioxidant Capacity (TAC) or protein- sulfhydryl group (-SH). However, significant relationships were found between TH and TAC, SH and -SH protein groups, and TAC and -SH protein groups- at the onset of and 24 hours after the phototherapy. Reduced levels of plasma bilirubin decreased plasma antioxidant capacity and increased levels of oxidative stress in preterm neonates. These could result from the pro-oxidant role of heme oxygenase due to the release of iron (an antioxidant property of bilirubin). Future studies are needed to examine preterm and term neonates separately and also with larger sample sizes 24.
Conclusion
The results of this study suggest that decreased levels of bilirubin after phototherapy cause a shift in the PAB values in favor of oxidants.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CCBY4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
AAP: American Academy of Pediatrics; NICU: Neonatal Intensive Care Unit ; PAB: Prooxidant Antioxidant Balance; STB: Serum Total Bilirubin
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no conflicts of interest.
Funding
No funding was received.
Authors' contributions
Hassan Boskabadi: Dr. Boskabadi conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Maryam Kalate Molaei : Kalate carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. Maryam Zakerihamidi: Dr Maryam Zakerihamidi designed the data collection instruments, and coordinated and supervised data collection at two of the four sites, critically reviewed the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
-
Boskabadi
H.,
Ashrafzadeh
F.,
Azarkish
F.,
Khakshour
A..
Complications of Neonatal Jaundice and the Predisposing Factors in Newborns. J Babol Univ Med Sci.
2015;
17
.
-
Maisels
M Jeffrey,
McDonagh
Antony F.
Phototherapy for neonatal jaundice. New England Journal of Medicine.
2008;
358
:
920-928
.
-
Maisels
M Jeffrey.
Phototherapy-Traditional and Nontraditional. Journal of perinatology.
2001;
21
.
-
Gathwala
Geeta,
Sharma
Seema.
Oxidative stress, phototherapy and the neonate. The Indian Journal of Pediatrics.
2000;
67
:
805-808
.
-
Boskabadi
Hassan,
Maamouri
Gholamali,
Omran
Farzaneh Rezagholizade,
Mafinejad
Shahin,
Tara
Fatemeh,
Rayman
Margaret P,
Ghayour-Mobarhan
Majid,
Sahebkar
Amirhossein,
Tavallaie
Shima,
Shakeri
Mohammad T.
Effect of prenatal selenium supplementation on cord blood selenium and lipid profile. Pediatrics & Neonatology.
2012;
53
:
334-339
.
-
Aycicek
Ali,
Erel
Ozcan.
Total oxidant/antioxidant status in jaundiced newborns before and after phototherapy. Jornal de pediatria.
2007;
83
:
319-322
.
-
Aycicek
Ali,
Kocyigit
Abdurrahim,
Erel
Ozcan,
Senturk
Hakan.
Phototherapy causes DNA damage in peripheral mononuclear leukocytes in term infants. Jornal de pediatria.
2008;
84
:
141-146
.
-
Scher
Mark.
Perinatal asphyxia: timing and mechanisms of injury in neonatal encephalopathy. Current neurology and neuroscience reports.
2001;
1
:
175-184
.
-
Jain
Sushil K.
The neonatal erythrocyte and its oxidative susceptibility. Seminars in hematology.
1989;
26
:
286-300
.
-
McDonagh
Antony F.
The role of singlet oxygen in bilirubin photo-oxidation. Biochemical and biophysical research communications.
1971;
44
:
1306-1311
.
-
Hulea
Stefan A,
Smith
Terrance L,
Wasowicz
Erwin,
Fred
Kummerow.
Bilirubin sensitized photooxidation of human plasma low density lipoprotein. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism.
1996;
1304
:
197-209
.
-
Perrone
Serafina,
Tataranno
Maria Luisa,
Stazzoni
Gemma,
Buonocore
Giuseppe.
Biomarkers of oxidative stress in fetal and neonatal diseases. The Journal of Maternal-Fetal & Neonatal Medicine.
2012;
25
:
2575-2578
.
-
Boskabadi
Hassan,
Mollaei
Maryam Kalate,
Zakerihamidi
Maryam,
Mobarhan
Majid Ghayour,
Bagheri
Fatemeh.
The effect of exchange transfusion on prooxidant-antioxidant balance in newborns Jaundice. Biomedical Research and Therapy.
2018;
5
:
2119-2129
.
-
Miller
Nicholas J,
Rice-Evans
Catherine,
Davies
Michael J,
Gopinathan
Vimala,
Milner
Anthony.
A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical science (London, England: 1979).
1993;
84
:
407-412
.
-
Stocker
Roland,
Yamamoto
Yorihiro,
McDonagh
Antony F,
Glazer
Alexander N,
Ames
Bruce N.
Bilirubin is an antioxidant of possible physiological importance. Science.
1987;
235
:
1043-1046
.
-
Tozzi
E,
Tozzi-Ciancarelli
MG,
Di Giulio
A,
D’Alfonso
A,
Farello
G,
Spennati
GF,
de Mattei
F.
In vitro and in vivo effects of erythrocyte phototherapy on newborns. Neonatology.
1989;
56
:
204-209
.
-
Boskabadi
Hassan,
Boroujeni
Abbas Navaee,
Mostafavi-Toroghi
Hesam,
Hosseini
Golkoo,
Ghayour-Mobarhan
Majid,
Alamdari
Dariush Hamidi,
Biranvandi
Mahtab,
Saber
Hamidreza,
Ferns
Gordon A.
Prooxidant-Antioxidant Balance in Perinatal Asphyxia. The Indian Journal of Pediatrics.
2014;
81
:
248-253
.
-
Surai
Peter F.
Natural antioxidants in poultry nutrition: new developments. 16th European Symposium on Poultry Nutrition.
2006;
:
669-676
.
-
Akisü
Mete,
Yilmaz
Deniz,
Tüzün
Sevgi,
Kültürsay
Nilgün.
Antioxidant defense systems in newborns undergoing phototherapy. The Indian Journal of Pediatrics.
1999;
66
:
651-655
.
-
Gopinathan
Vimala,
Miller
Nicholas J,
Milner
Anthony D,
Rice-Evans
Catherine A.
Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS letters.
1994;
349
:
197-200
.
-
Vreman
Hendrik J,
Wong
Ronald J,
Stevenson
David K.
Phototherapy: current methods and future directions. Seminars in perinatology.
2004;
28
:
326-333
.
-
Demirel
Gamze,
Uras
Nurdan,
Celik
Istemi H,
Aksoy
Hatice T,
Oguz
Serife S,
Erdeve
Omer,
Erel
Ozcan,
Dilmen
Ugur.
Comparison of total oxidant/antioxidant status in unconjugated hyperbilirubinemia of newborn before and after conventional and LED phototherapy: A prospective randomized controlled trial. Clinical & Investigative Medicine.
2010;
33
:
335-341
.
-
Dahiya
Kiran,
Tiwari
AD,
Shankar
Vijay,
Kharb
Simmi,
Dhankhar
Rakesh.
Antioxidant status in neonatal jaundice before and after phototherapy. Indian Journal of Clinical Biochemistry.
2006;
21
:
157-160
.
-
Dani
C,
Martelli
E,
Bertini
G,
Pezzati
M,
Filippi
L,
Rossetti
M,
Rizzuti
G,
Rubaltelli
FF.
Plasma bilirubin level and oxidative stress in preterm infants. Archives of Disease in Childhood-Fetal and Neonatal Edition.
2003;
88
:
F119-F123
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 7 (2018)
Page No.: 2432-2439
Published on: 2018-07-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8082 times
- Download PDF downloaded - 2431 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



