Abstract
Background: Estimating total, direct, and indirect effects of causal variables on outcome variables can be done through proposed mediator guides on causal pathways. This can improve the efficiency of diagnostic efforts done by clinicians. In this study, the relationship between Sulfur Mustard and Xerosis was investigated, specifically with respect to the role of Hemato_4, Hemato_5, Hemato_9, and Biochem_20 biomarkers as mediators.
Methods and Results: This was a historical cohort study (in Sardasht, Iran) which consisted of 492 subjects; 129 subjects were not exposed to Mustard Gas (control group) and 363 subjects were exposed to it (case group). Mediation models along with bootstrap method were used to evaluate the mediator validity of Hemato_4, Hemato_5, Hemato_9, and Biochem_20 on the relationship between Sulfur Mustard and Xerosis. The direct effects of Sulfur Mustard, via Hemato_4 and Hemato_9, on Xerosis were also significant (P<0.05 for each). However, there was no significant effects mediated by Hemato_5 or Biochem_20 (P>0.05 for each). While there were non-significant indirect effects of Sulfur Mustard on Xerosis by these latter biomarkers (P>0.05 for each), the first two biomarkers were, indeed, partial mediators.
Conclusion: Sulfur Mustard can affect Xerosis through several single immune biomarker. However, as an intervention, sulfur mustard should affect several biomarkers through various mechanisms. Therefore, effects through multiple mediators, instead of single ones, may be more rational in the treatment strategy for xerosis.
Background
Sulfur mustard (SM) is a cytotoxic, blistering, and irritant military chemical agent capable of reacting with most biologic molecules of the human body. While it is not a gas, it is a semi- liquid chemical capable to turning into steam. It is chlorosis in its pure form but unstable and carcinogenic when used as a military agent [1]. Upon contact with soil and water, SM evaporates in the air and settles down in minutes to days, depending on the environmental conditions. Since infection signs do not appear immediately and contaminated areas look quite normal, exposed individuals may receive large doses of it [2][3].
Its main acute pathological complications are depigmentation and wet lesions on the skin, lung function disturbances, ophthalmologic manifestations, conjunctivitis, corneal abrasion gastrointestinal symptoms, hematological complications, and bone marrow failures. One of the most catastrophic effects of SM poisoning is suppression of the immune system which leads to lesions and blisters, and results in opportunistic infections [4]-[7]. Slight or moderate exposure is not usually fatal but a long period of treatment is needed; even after remedy for the burns, there is still a great risk of developing cancer. SM and its complications for the human body can be detected with blood and urine tests within a few weeks after the last exposure [2]. It is found in the fat of thigh, abdominal skin, and subcutaneous fat. Lung, spleen, liver, blood, and urine specimens also show low concentrations of unmetabolized SM in patients [8].
Xerosis, or dry skin, refers to sensitive skin which is easily irritated by external stimuli and is, most importantly, itchy. It is often located on the extensor surface of the legs and arms, but in susceptible individuals, it may involve the entire cutaneous surface. Also, it is worse in the winter when humidity is low. Water is lost from the outer most layer of the skin [8]. Dry areas that are repeatedly washed reach a point at which the epidermal barrier can no longer maintain its integrity. Dry skin is an important feature of an atopic state whose characteristics vary with age.
Atopic dermatitis seems to develop from a vicious circle of dermatitis associated with elevated T-lymphocyte activation, hyperstimulatory Langerhans cells, defective cell-mediated immunity, and B-cell mediated IgE overproduction. Triggering factors include temperature change and sweating, decreased humidity, excessive washing, contact with irritating substances, contact allergy, aeroallergens, antimicrobial agents, food, and emotional stress [5][9].
Using Path analysis, the effects of the relationship between exposure and outcome, as impacted by mediators, can be assessed. Path analysis was first used in 1920 for quantitative genetic studies and later in ecology, psychology, social sciences and economics [10][11], as well as health sciences [11]-[13]. It helps to illuminate the relative strength of direct and indirect relationships among a variety of variables. In this statistical approach, a set of causes and effects were diagnosed as a series of steps in a path with a coefficient assigned to each step for quantifying the relationships [14]-[16]. Moreover, Path analysis can be an effective tool to identify the main risk factors of earlier biological effects [17]. Fourteen different methods have been proposed for testing models including intervening variables, and according to them, three general approaches have been offered. These include causal steps in which 4 criteria are tested in the causal step methods [18]-[19]. Due to MacKinnon et al., the independent variable has both direct and indirect effects on the dependent variable (through a mediating variable), if these effects are equal in magnitude but opposite in sign.
The next component is ‘difference in coefficients’, based on comparing two regression or correlation coefficients, for the relationship between independent and dependent variables (skipping mediating variable) and for the relationship between independent and dependent variables after removing the effect of the mediating variable on the dependent variable. Finally, the ‘indirect effect’ of the independent variable on dependent variable, through a mediator, is obtained by multiplying the coefficient for path independent variable-mediator by the coefficient for path mediator-dependent variable [20][21].
Basic and clinical studies have been done on immediate and long-term effects of SM in both animals and human beings [21]-[23], mostly on respiratory and pulmonary systems [24]-[26], skin [27][28], eyes [29], immune system [30][31], urologic and physical conditions of exposed individuals [32][33], and alterations in the serum levels of cytokines, chemokines, immunoglobins, and testosterone [34][35].
Using a Sardasht-Iran historical cohort study (1), the aim of this research was to assess Hemato_4 (HCT), Hemato_5 (PLAT (1000)), Hemato_9 (MCHC), and Biochem_20 (Alkaline Phosphatase; ALK.Phosphat IU/L (IFCC)) as biomarkers and possible mediators of the relationship between Xerosis and SM.
Methods
Study design/ area
During the Iraq-Iran war (1980-1988), Sulfur Mustard was extensively used against Iran as a chemical weapon. To date, more than 100,000 Iranians- military veterans and civilians- have suffered from its short and long-term complications. Sardasht-Iran Cohort study is a comprehensive historical cohort study which aims to explore the basic mechanisms underlying clinical complications caused by SM in Sardasht residents 20 years after exposure [1] . Initiated in June 2006, it is still ongoing. Health status, long-term clinical complications, local immune responses, hematologic and biochemical parameters, along with lifestyle of chemical victims, are evaluated in the SM-exposed and unexposed groups.
Sample size
Among the variables of this study, immunoglobulins have more variations and, thus, the sample size was calculated with immunoglobin G (IgG) levels. The mean±SD of IgG in chemically exposed veterans (n=40) and control group (n=35) were 1438.6±481.1 and 1140.0±244.2 (mg/mL), respectively. Thus, the 95% confidence interval (CI) for IgG difference (µ2-µ1) was 127.6 to 469.6. In addition, a 95% CI for variance proportion (δ2/δ2) was found to be 2.1 to 4.7. Since the variances in the two groups were not equal, sample sizes were unbalanced; the exposed group may have more participants than the control group. For a type I error ( of 0.05, power of analysis of 0.95, type II error (β) of 0.05, and a variance proportion (k=δ2/δ2) of 3, the lower band of the CI was chosen as the difference between two groups (6 = µ2 − µ1), i.e., 127.6. The sample size of the two groups, F1 and F2, were 363 and 129, respectively, for case and control groups (1).
n1 = (s21/k + s22)x (φ (1 − α/2) + φ (1 − β))2/▵2 (F1)
n2 = (s21 + ks22)x (φ(1 − α/2) + φ (1 − β))2/▵2 (F2)
Subjects
The exposed group was selected from the male individuals of Sardasht, Iran who were exposed to SM, based on documents in the Medical Committee of the Foundation of Martyrs and Veterans’ Affair. In the medical records, each victim had a medical file with a numeric code. The exposed subjects were selected by systematic random sampling method from the chemical victims’ list; since the ratio of sample size to records was 0.1, each sample was selected from every 10 records. The non-exposed group was selected from Rabat, the town located 15 kilometers away from Sardasht. Both towns had similar geographical situations and weather conditions. The populations were Kurdish, with the same religion, culture, language, and nutritional habits. Their only difference was exposure to SM. The non-exposed group individuals were males selected by systematic random sampling from the list of latest demographic households’ statistics at the local health center [1].
Participants were matched by age based on the inclusion and exclusion criteria as summarized below.
Inclusion criteria
SM exposure in June 1987 based on medical records (for exposed group), age of 20- 60 years, informed consent, and no systematic immunosuppressive medication.
Exclusion criteria
Age<20 and >60 years, current treatment with systematic immunosuppressive drugs, history of systematic disease before exposure (based on medical records), suffering from an acute infectious disease at the time of sampling, and disinclination to continue participation.
Variables were collected from the four following sources: data in medical records, questionnaire variables, clinical evaluation, and laboratory procedures and specimen collection. The study group is a binary variable (0 for no exposed and 1 for exposed); Xerosis is a binary variable (0 for free of Xerosis and 1 for having Xerosis); and all biomarkers (Hemato_4, Hemato_5, Hemato_9, and Biochem_20) are continuous [1]. Total sample size, sample size of each study, age distributions, and demographic information of the population under study (such as Hemato_4, Hemato_5, Hemato_9, and Biochem_20 biomarkers) were assessed.
Questionnaire variables
All the participants were evaluated for their socio-demographic information, medical and family history, medications, anthropometric measurements, resting blood pressure, heart rate, and any sign of acute infectious diseases. Standard questionnaires related to psychosocial factors, lifestyle, sleep quality, and healthcare services were administered by clinical psychologists, health educators, and nurses [1].
Statistical analysis/ information processing
In statistical tests which include M (mediating variables), X (independent variables) is correlated with Y (dependent variables). This is not due to the direct effect that the mediating variable exerts on the dependent variable, but because it causes a change in a mediating variable, which in turn causes a change in the dependent variable. In a mediating model, X should be correlated with Y, X should be correlated with M (holding constant any direct effect of X on Y), and M should be correlated with Y. Moreover, if the effect of M on Y is omitted, X is no longer correlated with Y (complete mediation) or correlation is reduced (partial mediation). Generalized Linear Model and X2 tests were employed to investigate the involvement of HCT, PLAT (1000), MCHC, and Alkaline phosphatase (ALK.Phosphat) IU/L (IFCC) as possible mediators of the relationship between SM and Xerosis using R-2.6.0 statistical software (company, location?)
Mediation modeling
Direct, indirect, and total effects were all obtained from the path coefficients stated in the mediating models. Total effect is decomposed to direct and indirect effect. The coefficient for indirect effect of X on Y through M is obtained by multiplying the coefficient for path XM by the coefficient for path MY. The coefficient for path XM is either the significant correlation between X and M or beta weight of regression predicting M from X. The coefficient for path MY is the beta weight for M from the multiple regression predicting Y from X and M. Stated coefficients can be both standardized and UN standardized coefficients. For testing mediator validity of related biomarkers, the test statistic is computed by dividing indirect coefficient by its standard error.
To assess the accuracy of the effects, the bootstrap method is applied. In this method, using repeated random samples, variance and bias of the effects are computed (with maximum likelihood approaches). If the effects obtained from GLMs are close to the effects obtained from the bootstrap model, resulting in partial bias, then the proposed effects are accurate ones. The mediator is a quantitative variable, the independent variable and the dependent variable can be quantitative or binary, but the mediator should not be dichotomous. A case will be deleted if missing on any of the variables in the model are missing. Kris Preacher’s ’SAS and SPSS macro for mediation analysis” was used for identifying direct and indirect effects. Overlay was done; α was considered 0.05 and all tests were two tailed.
Results & Discussion
Assuming thatY is Xerosis (present vs. absent), X is study group (exposed to SM vs. non- exposed to SM), and M is mediator (HCT, PLAT (1000), MCHC or ALK. Phosphat IU/L (IFCC) biomarkers) using mediation models, the total, direct and indirect effects of variables using the bootstrap method are indicated in Table 1 , Table 2 , Table 3 and Table 4 . Each shows the accuracy of all effects obtained. The coefficient, significance, and variance are close to that gained from GLMs.
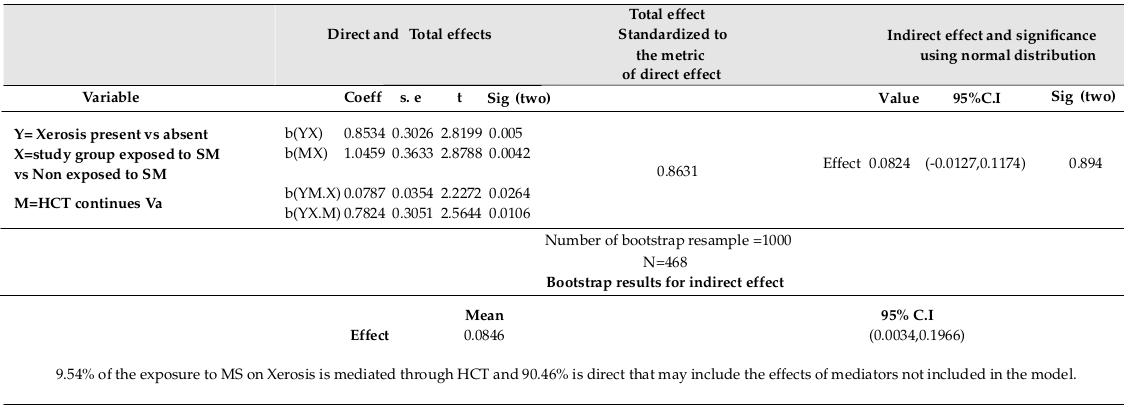
As expected, Xerosis was significantly related to study group (χ2=9.489, p<0.005). HCT was found to be significantly related to study group (R=0.132, F (1,466) =8.287, p<0.0042 (using linear regression, β=1.046, p<0.0042)). Xerosis was significantly related to HCT (using logistic regression, Exp (β) =1.093, p=0.011). Xerosis was significantly related to the combination of HCT and study group (using logistic regression, Exp (β) =1.082, p=0.026, Exp (β) =2.187, p=0.01 respectively). Direct and indirect effects of Exposure on Xerosis explained by HCT are shown in Table 1 .
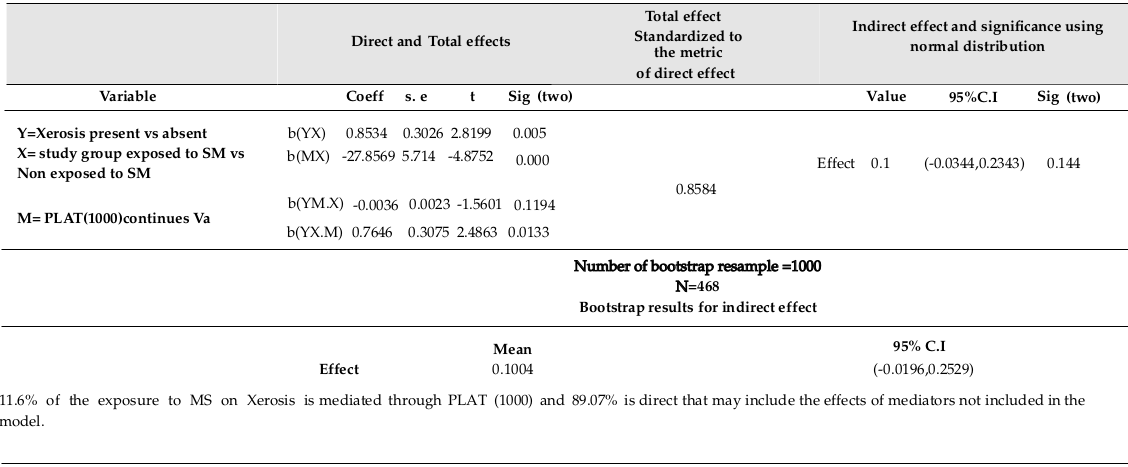
PLAT (1000) was found to be significantly related to study group (R=0.22, F (1,466) =23.767, p=0.000) using linear regression (β=-27.857, p=0.000). Xerosis was significantly related to PLAT (1000) (using logistic regression Exp (β) = 0.995, p=0.036). Xerosis is not significantly related to the combination of PLAT (1000) and study group (using logistic regression; Exp (β) =0.996, p=0.119, Exp (β) =0.013, p=0.013, respectively). Direct and indirect effects of Exposure on Xerosis explained by PLAT (1000) are shown in Table 2 .
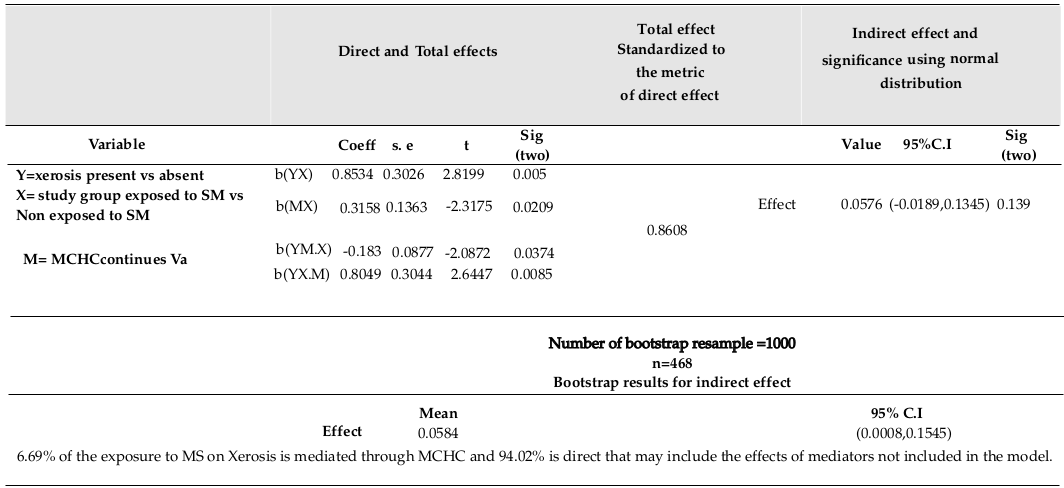
MCHC was found to be significantly related to study group (R=0.107, F (1,466) = 5.371, p=0.021; using linear regression, β=-0.316, p=0.021). Xerosis was significantly related to MCHC (using logistic regression, Exp (β) =0.817, p=0.019). Xerosis was significantly related to combination of MCHC and study group (using logistic regression, Exp (β) =0.833, p=0.037, Exp (β) =2.236, p=0.008, respectively). Direct and indirect effects of Exposure on Xerosis are explained by MCHC, as shown in Table 3 .
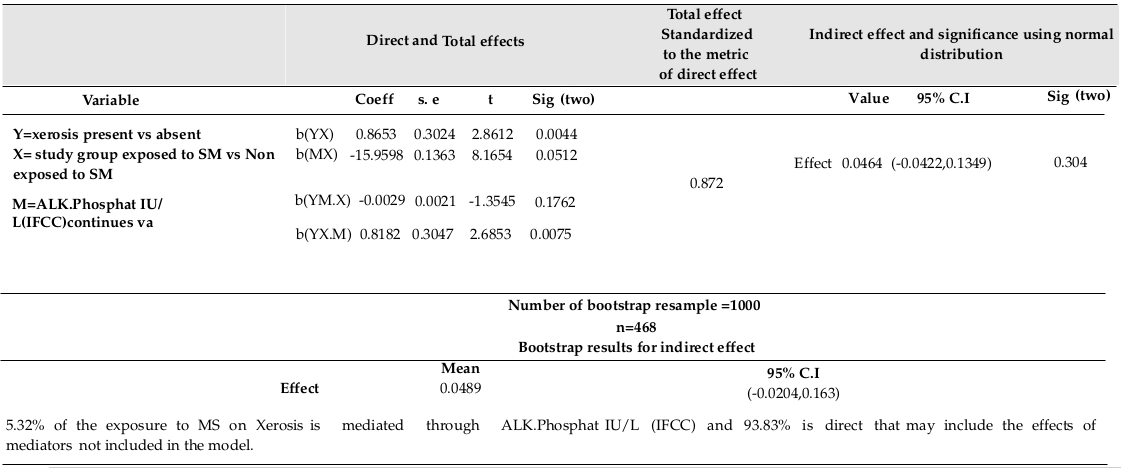
ALK.Phosphat IU/L (IFCC) was not found to be significantly related to the study group (R=0.09, F (1,467) = 3.82, p=0.051; using linear regression, β=-15.96, p=0.051). Xerosis was not significantly related to ALK.Phosphat IU/L (IFCC) (using logistic regression Exp (β) = 0.996, p=0.08). Xerosis was not significantly related to combination of ALK.Phosphat IU/L (IFCC) and study group (using logistic regression Exp (β) =0.997, p=0.176, Exp (β) =2.267, p=0.007, respectively). Direct and indirect effects of Exposure on Xerosis, as explained by ALK.Phosphat IU/L (IFCC), are shown in Table 4 .
It is more rational to have a set of biomarkers as a potential set of mediators or multiple mediators. Using bootstrap of 1000 at α= 0.05 showed significant indirect effects of SM on Xerosis mediated through two biomarkers (HCT and MCHC), with increased false positive error. Indeed, 90.46% of the effect of SM on Xerosis is direct without being mediated through HCT. This effect could have been mediated through other biomarkers not included in the model. In the pathway of SM on Xerosis, some other important mediating variables should be considered and assessed as better mediators- PLAT (1000), MCHC, and ALK.Phosphat IU/L (IFCC).
SM may not have any direct effect on Xerosis, but it can impose indirect effects through HCT and other biomarkers. Indeed, if these biomarkers are not included in the model, then the indirect effect of SM on Xerosis may be mistaken as a direct effect of SM on Xerosis [24].
Conclusion
The bootstrap method showed valid computations, in addition to the 9.54%, 11.6%, 6.69%, and 5.32% of the mustard sulfur (MS effects) on Xerosis, as explained by HCT, PLAT (1000), MCHC, and ALK-Phosphat IU/L (IFCC), respectively. The partial mediating effects of HCT and MCHC are also included. Indeed, all variables considering in a multiple mediating model could result in better mediation validity since SM affects several organisms at the same time.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited
List of abbreviations
SM: Sulfur Mustard
Competing interests
The authors declare no conflict of interest.
Funding
Department of Biostatistics, Tarbiat Modares University, Tehran, Iran.
Ethics approval and consent to participate
This study, based on (part of a cohort data), is a cohort study, available on the website of the Iranian Ministry of Health. Notice the reference number one that points to this issue.
Availability of data and material
Will be provided if request.
Author Contribution
All authors contributed to the design of the research. MZ, SF, SJ and MS collected the Data. ZKH, MZ and SF conducted analysis and interpretation of data. All authors drafted the first version. MZ, SF, SJ, and MS edited the first draft. All authors reviewed, Commented and approved the final draft.
References
-
H
Aragizadeh,
MR
Soroush,
MA
Javadi,
F
Azizi,
H
Ghasemi,
J
Shams,
S
Pourfarzam,
F
Fallahi,
SM
Davoudi,
S
Shariat-Panahi.
Sardasht-Iran cohort study of chemical warfare victims: design and methods. Archives of Iranian medicine.
2009;
12
:
5-14
.
PubMed Google Scholar -
null
null.
Agency for toxic substances and disease registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs),(update) PB/95/264370. Atlanta: US Department of Health and Human Services.
1995
.
-
M
Meselson,
JP
Robinson.
Chemical warfare and chemical disarmament. Scientific American.
1980;
242
:
38-47
.
View Article Google Scholar -
G
Pauser,
A
Aloy,
M
Carvana,
W
Graninger,
M
Havel,
W
Koller,
N
Mutz.
Lethal intoxication by wargases on Iranian soldiers. Therapeutic interventions on survivors of mustard gas and mycotoxin immersion. Archives belges= Belgisch archief.
1984;
:
341-351
.
-
H
Sohrabpour.
Observation and clinical manifestations of patients injured with mustard gas. Medical Journal of The Islamic Republic of Iran (MJIRI).
1987;
1
:
32-37
.
-
W
Vycudilik.
Detection of mustard gas bis (2-chloroethyl)-sulfide in urine. Forensic science international.
1985;
28
:
131-136
.
View Article Google Scholar -
M
Balali-Mood,
B
Balali-Mood.
Sulphur mustard poisoning and its complications in Iranian veterans. Iranian Journal of Medical Sciences.
2015;
34
:
155-171
.
-
E
Darchini-Maragheh,
M
Balali-Mood.
vDelayed Complications and Long-term Management of Sulfur Mustard Poisoning: Recent Advances by Iranian Researchers (Part of). Iranian Journal of Medical Sciences.
2018;
43
:
103-124
.
PubMed Google Scholar -
Y
Panahi,
M
Naderi,
S
Nekoozadeh,
SM
Rajaee,
A
Sahebkar.
The Effectiveness of Different Treatment Modalities for the Management of Ocular Injuries Following Sulfur Mustard Exposure. Letters in Drug Design & Discovery.
2018;
15
:
203-211
.
-
WBL.
M.
Genetics and data analysis of quantitative trails. Sunderland (MA).
1998
.
-
B
Shipley.
Exploratory path analysis with applications in ecology and evolution. The American Naturalist.
1997;
149
:
1113-1138
.
View Article Google Scholar -
JR
GOLDSMITH.
Paths of association in epidemiological analysis: application to health effects of environmental exposures. International journal of epidemiology.
1977;
6
:
391-399
.
View Article Google Scholar -
L
Qiu,
S
Leng,
Z
Wang,
Y
Dai,
Y
Zheng,
Z
Wang.
Path analysis of biomarkers of exposure and early biological effects among coke-oven workers exposed to polycyclic aromatic hydrocarbons. Cancer Epidemiology and Prevention Biomarkers.
2007;
16
:
1193-1199
.
View Article Google Scholar -
DD
Kay,
GMBJ
Lee KS.
Biosocial pathways to functional outcome in schizophrenia. a. Schizophr Res..
2005;
8
:
213-225
.
-
CAF
Santos,
D
Senalik,
PW
Simon.
Path analysis suggests phytoene accumulation is the key step limiting the carotenoid pathway in white carrot roots. Genetics and Molecular Biology.
2005;
28
:
287-293
.
View Article Google Scholar -
B
Shipley.
Cause and correlation in biology: a users´ guide to path analysis, structural equations and causal inference with R. 2016;
:
nll
.
-
CH
Brooks.
Path analysis of socioeconomic correlates of county infant mortality rates. International Journal of Health Services.
1975;
5
:
499-514
.
View Article Google Scholar -
DP
MacKinnon,
CM
Lockwood,
JM
Hoffman,
SG
West,
V
Sheets.
A comparison of methods to test mediation and other intervening variable effects. Psychological methods.
2002;
7
:
83
.
View Article Google Scholar -
RM
Baron,
DA
Kenny.
The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of personality and social psychology.
1986;
51
:
1173
.
View Article Google Scholar -
PE
Shrout,
N
Bolger.
Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological methods.
2002;
7
:
422
.
View Article Google Scholar -
ME
Sobel.
Asymptotic confidence intervals for indirect effects in structural equation models. Sociological methodology.
1982;
13
:
290-312
.
View Article Google Scholar -
M
BALALIMOUD,
M
Hefazi.
The clinical toxicology of sulfur mustard. Archives of Iranian medicine.
2005
.
-
M
Balali-Mood,
M
Hefazi,
M
Mahmoudi,
E
Jalali,
D
Attaran,
M
Maleki,
ME
Razavi,
G
Zare,
A
Tabatabaee,
MR
Jaafari.
Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundamental & clinical pharmacology.
2005;
19
:
713-721
.
View Article Google Scholar -
ZT
ROUSHAN,
N
MIRKHESHTI,
F
GHASEMI,
MN
AFSHAR,
S
ALAVI.
Bcl-2 protein expression in pulmonary specimens of sulfur mustard victims. 2008
.
-
A
Emad,
GR
Rezaian.
Characteristics of bronchoalveolar lavage fluid in patients with sulfur mustard gas-induced asthma or chronic bronchitis. The American journal of medicine.
1999;
106
:
625-628
.
View Article Google Scholar -
M
Shohrati,
M
Ghanei,
N
Shamspour,
M
Jafari,
J
Aslani.
Effect of long-term pulmonary Complications due to sulfur mustard on activation of enzymes in antioxiadant system. Trauma Monthly.
2008;
2008
:
65-70
.
-
ABZ
Mahmoudabad,
M
Saberi,
J
Pirzad.
Critical role of GSH in sulfur mustard- induced oxidative stress and cytotoxicity in human skin fibroblast cell line. Iranian Journal of Pharmaceutical Research.
2010;
:
35-41
.
-
SD
DAVOUDI,
S
Keshavarz,
B
SADR,
M
Shohrati,
MM
Naghizadeh,
KH
FARSINEZHAD,
FM
RASHIGHI,
H
Zartab,
AR
FIROUZ.
Comparison of skin erythema and melanin level in sulfur mustard induced chronic skin lesions and normal skin. 2008
.
-
H
Ghasemi,
T
Ghazanfari,
R
Yaraee,
M
Ghassemi-Broumand,
MR
Soroush,
S
Pourfarzam,
Z
Masdari,
S
Faghihzadeh,
M
Babaei,
MA
Javadi.
Evaluation of relationship between the serum levels of inflammatory mediators and ocular injuries induced by sulfur mustard: Sardasht-Iran Cohort Study. International immunopharmacology.
2009;
9
:
1494-1498
.
View Article Google Scholar -
A
Keyhani,
MB
Eslami,
H
Razavimanesh.
The short-term effect of mustard gas on the serum immunoglobulin levels. Iranian Journal of Allergy, Asthma and Immunology.
2007;
6
:
15-20
.
PubMed Google Scholar -
L
Ghotbi,
Z
Hassan.
The immunostatus of natural killer cells in people exposed to sulfur mustard. International immunopharmacology.
2002;
2
:
981-985
.
View Article Google Scholar -
MR
Soroush,
M
Ghanei,
S
Assari,
HRK
Vishteh.
Urogenital history in veterans exposed to high-dose sulfur mustard: a preliminary study of self-reported data. Urology journal.
2009;
6
:
114-119
.
PubMed Google Scholar -
Z
Ghazanfari,
T
Ghazanfari,
R
Yaraee,
R
Amini,
S
Ghaderi,
A
Pirasteh,
S
Moaiedmohseni,
M
Naghizadeh,
S
Faghihzadeh.
Aassociation between physical activity and body mass index in the civilian chemical victims of Sardasht 20 years after sulfur mustard exposure. Iranian Journal of War and Public Health.
2009a;
1
:
1-8
.
-
T
Ghazanfari,
R
Yaraee,
A
Kariminia,
M
Ebtekar,
S
Faghihzadeh,
MR
Vaez-Mahdavi,
A
Rezaei,
M
Vojgani,
MR
Soroush,
A
Kermani-Jalilvand.
Alterations in the serum levels of chemokines 20 years after sulfur mustard exposure: Sardasht-Iran Cohort Study. International immunopharmacology.
2009b;
9
:
1471-1476
.
-
S
Pourfarzam,
T
Ghazanfari,
R
Yaraee,
H
Ghasemi,
ZM
Hassan,
S
Faghihzadeh,
SK
Ardestani,
A
Kariminia,
F
Fallahi,
MR
Soroush.
Serum levels of IL-8 and IL-6 in the long term pulmonary complications induced by sulfur mustard: Sardasht-Iran Cohort Study. International immunopharmacology.
2009;
9
:
1482-1488
.
View Article Google Scholar
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 6 (2018)
Page No.: 2402-2413
Published on: 2018-06-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5671 times
- Download PDF downloaded - 1784 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress