Abstract
Background: Transplantation of adipose-derived stem cells (ADSCs) is a potential therapy for a variety of liver diseases. Previous studies have shown that ADSC-based therapy is promising for liver fibrosis treatment. Recently, many publications have suggested that pretreating ADSCs with growth factors before transplantation can elevate the effectiveness of the therapy. Therefore, we hypothesize that human ADSCs (hADSCs) pretreated with platelet-rich plasma (PRP) and hepatocyte growth factor (HGF), compared to ADSCs alone, would accelerate the treatment effects on liver fibrosis in mice.
Methods: The hADSCs were cultured solely with conventional media, or with HGF (human recombinant; 20 ng/ml), or with HGF and PRP (from healthy human blood, 10%), concomitantly added to the medium for 7 days before transplantation. Eight-week-old male mice were treated with CCl4 (1 ml/kg) for 11 weeks to induce liver fibrosis. The mice were then subsequently divided into: 1) Placebo group (PBS injection); 2) ADSCs/HGF+PRP (5x105HGF and PRP pre-treated cells/mice); 3) ADSCs/HGF (5x105HGF pre-treated cells/mice) and 4) ADSCs (5x105non-pretreated cells/mice).
Results: Seven days post-transplantation, the alanine transaminase (ALT) level in the placebo was notably elevated (143.10±14.96 IU/L), compared to ALT levels of ADSCs-, ADSCs/HGF+PR-, and ADSCs/HGF-transplanted mice, which showed an improvement (67.94±18.57 IU/L, 49.44±7.56 IU/L, and 57.93±5.75 IU/L, respectively). The procollagen-α1 and alpha-smooth muscle actin (α-SMA) mRNA levels were significantly down-regulated in the ADSCs-transplanted group compared to those of the placebo. Importantly, these levels were lower in ADSCs/HGF+PR (procollagen-α1: 75.64±45.89; α-SMA: 36.17±36.09) than those of ADSCs/HGF (procollagen-α1: 212.8±84.35; α-SMA: 52.41±7.93) and ADSCs only (procollagen-α1: 310.50 ± 55.36; α-SMA: 184.70±14.06). Stem cell transplantation also improved histological index, reducing inflammation and collagen/necrotic structure accumulation. However, there were no statistical differences between three ADSCs treatment groups after 14 days after transplantation.
Conclusion: Pre-treatment with PRP and HGF for 7 days enhanced the efficacy of ADSCs in alleviating liver fibrosis in vivo.
Introduction
According to the World of Health Organization (WHO), liver fibrosis/cirrhosis is one of the major global burdens. In 2010, liver fibrosis caused 1 million deaths and occupied 2% of total mortality cases in the world [1]. Additionally, liver fibrosis has also been known as one of the leading causes of hepatic carcinoma. Currently, orthotopic liver transplantation is a gold therapeutic standard for liver fibrosis as it is a life-prolonging treatment [2]. In recent years, mesenchymal stem cell (MSC)-based therapy for liver fibrosis has gained increased interest due to proliferative ability of MSCs in vitro, and abundance of MSC source origins, which include adipose tissue [3]–[5], bone marrow [6][7] , and umbilical cord blood [8][9]. Moreover, the paracrine effects and differentiation capacity of MSCs are beneficial [10]. Indeed, MSC-based therapy has attained promising results in liver fibrosis treatment [cite].For regenerative medicine, many research studies are now focusing on ways to improve the impact of MSCs- on cell survival, homing, differentiation capability and therapeutic efficacy. Many approaches have been investigated with the aim to enhance MSC efficacy by pre-treatment with cytokines and growth factors [11], using 3D-scaffolds for MSC culture [12] and assessing gene modifications [13]. In this study, we hypothesized that pre- treatment with platelet-rich plasma (PRP) and hepatocyte growth factor (HGF) may augment the effect of adipose-derived stem cells (ADSCs) on liver fibrosis treatment in mice. HGF is known as a crucial factor for hepatocyte division [14][15]. Previous publications indicate that HGF is an essential ligand for development and regeneration of hepatocytes [16]–[18]. Moreover, it considered as a cytokine with abundant roles, such as cell division stimulator and morphogenesis factor [16][19], HGF has been a significant growth factor in research and clinical applications, and plays a crucial role in stimulating endogenous MSCs to move to sites of injury [20]. Indeed, published data have shown that HGF in culture improves the homing capability of MSCs [21]-[23]. Over-expression of HGF gene in MSCs enhanced the therapeutic effect of MSCs in a liver fibrosis mouse model . PRP is defined as the plasma which encompasses a high concentration of platelet- derived growth factors (> 3-5 fold greater than whole blood) [24]. PRP contains an abundance of essential growth factors, including epidermal growth factor (EGF), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin growth factor (IGF), and Keratinocyte Growth Factor (KGF) [25][26]. Presently, PRP has been investigated in applications for at least 30 diseases [26]. Studies have demonstrated that in medium supplied with PRP, MSCs showed an increase in proliferation rate, stemness [27] and immunomodulatory ability [28]. Notably, recent data showed that culturing with PRP enhanced the homing and immunomodulatory capacity of MSCs [29][30].
Methods
Induction of fibrosis in mice
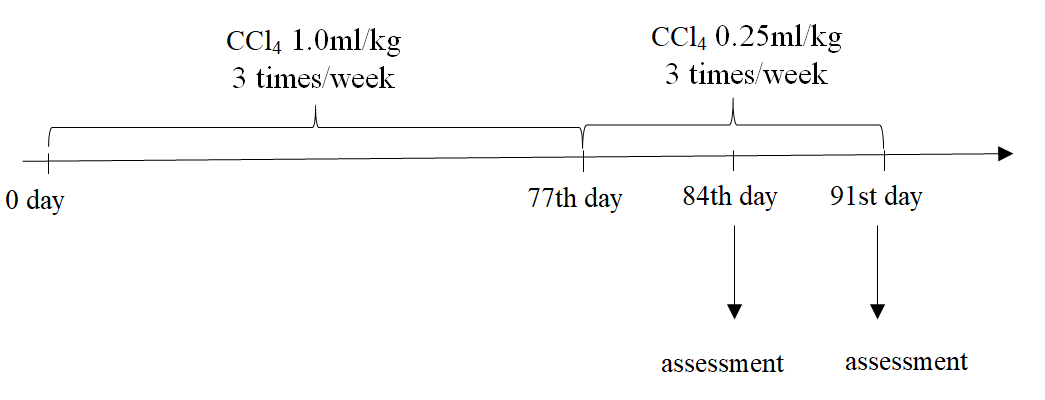
The protocol for inducing fibrosis in mice was followed as previous publication [31]. In brief, healthy 8-week old male Swiss mice (weight of 25-28 g) were treated by 1.0 mL/kg CCl4 (99.5% purity, UNI-CHEM Chemical Reagent, China) via oral administration three times per week (every two days) for 11 consecutive weeks. Mice fasted for 4 hours before oral administration of CCl4. This study was accepted by the Ethical Committee of Stem Cell Institute, University of Science, Vietnam National University of Ho Chi Minh City (VNU-HCMUS) in Viet Nam.
Culture of human adipose-derived stem cells (hADSCs)
Frozen human adipose-derived stem cells (hADSCs) at passage 3 (Stem Cell Institute) was thawed using Thawbest medium (Gene World. Co. Ltd, Ho Chi Minh City, Vietnam). Cells were cultured in Dulbecco’s modified Eagle’s medium/F12 (Sigma-Aldrich, St. Louis, MO), 1X antibiotic- antimycotic (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS, EU Thermo Scientific, MA); or with HGF (20 ng/ml, Biolegend); or with HGF (20 ng/ml) + PRP 10%; cells were named as ADSCs, ADSCs/HGF and ADSCs/HGF+PRP, respectively.
Characterization of hADSCs
Cell marker surface was analyzed by the FACS-Calibur machine (BD Biosciences, San Jose, CA) using FITC- or PE-conjugated anti- CD34, anti-CD45, anti-CD14, anti-CD44, anti-CD90, or anti- CD166 antibodies. The data were analyzed by FlowJo software (FlowJo LLC, Ashland, OR).
For adipogenic and osteogenic differentiation, cells were induced as previously described [32]. In brief, cells were cultured in DMEM/F12 medium (Sigma Aldrich, Louis, MO) supplemented with 10% FBS, 1X antibiotic-antimycotic solution, 10 mM dexamethasone (Sigma Aldrich), 2.79 mM indomethacin (Sigma Aldrich), 5 mg/mL insulin (Sigma Aldrich), and 0.5 M 1-Methyl- 3- isobutylxanthine (IBMX) (Sigma Aldrich) for adipogenic differentiation. Cells were stained with Oil Red O (Sigma Aldrich) to determine lipid droplet at day 21 of the differentiation.
For osteogenic differentiation, cells were induced by medium supplemented with ascorbic acid, dexamethasone, and 6-glycerol phosphate (all purchased from Sigma-Aldrich). Calcium deposits were visualized by staining with Alizarin Red (Sigma Aldrich).
Cell proliferation assay
The proliferation of cells was determined by MTT method. After 24 hours of culture, 20 µl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; 5 g/l; Sigma-Aldrich) was added to the culture medium of each well. The plates were incubated in the dark for 5 minutes and then, 50 µl of medium was discarded and 150 µl of DMSO (Sigma-Aldrich) added to each well. Absorption values were measured after 48, 72 and 96 hours at a wavelength of 595 nm. Doubling time was calculated by the following formula:
DT = tlog(2)/(log(ODt)−log(ODto))
ADSC transplantation
Fibrosis-bearing mice were divided into four groups (n = 5 each group): Placebo group (PBS injection); ADSCs/HGF+PRP (5x105 HGF and PRP pre-treated cells/mice); ADSCs/HGF (5x105 HGF pre-treated cells/mice) and ADSCs ((5x105 non-pretreated cells/mice).
At 80% confluence, the ADSCs were obtained by using 0.25% trypsin/EDTA (Sigma-Aldrich) then washed in PBS. The ADSCs were filtered using 70 µm pore size filter membranes (BD Bioscience). Then, 5x105 cells were suspended in 150 µl PBS and injected into mice via tail vein.
Biochemical Assessments
At 7 and 14 day post-transplantation, mice have examined the improvement of serum aspartate transaminase (AST), alanine transaminase (ALT), and albumin (ALB) levels. Then, 200 µl of serum from mice was collected for AST (IFCC Mod.LiquiUV test, Germany), ALT (IFCC Mod. LiquiUV test, Germany) and ALB (QuantiChrom Bilirubin Assay Kit, Bioassay Systems, CA, USA) measurements, according to the manufacturers’ instructions.
Quantitative reverse transcription PCR (RT-qPCR)
Expression of fibrosis-related genes
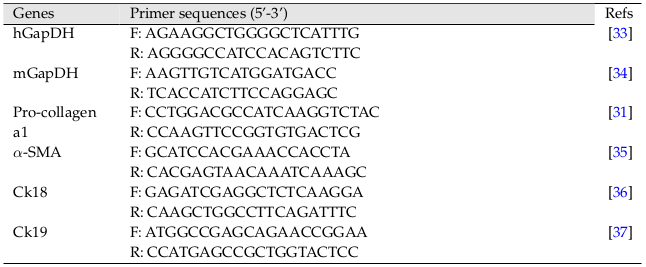
The partial left liver lobes of mice from each group were collected. Total RNA was isolated using Easy-BLUE Total RNA Extraction Kit (INTRON Biotechnology, Korea) and then evaluated for the expression of genes by RT-qPCR method (Brilliant II QRT-PCR Master Mix kit 1-step, Agilent, CA) using the primers listed in Table 1 .
Expression of CK18 and CK19
HGF and PRP pre-treated cells were used to isolated total RNA using Easy-BLUE Total RNA Extraction Kit (INTRON Biotechnology) and then evaluated for expression of genes using the respective specified primers listed in Table 1 .
Histological examination
Liver tissues (approximately 1 cm2) were obtained from left liver lobes. The samples were washed with PBS twice and fixed in paraformaldehyde (Merck Millipore, Germany) for 24 hours before sectioning in paraffin.
For hematoxylin & eosin (H&E) staining, liver sections were deparaffinized in xylene, dehydrated using alcohol, and washed with PBS. Slides were then stained with hematoxylin (Merck Millipore, Germany) for 5 min, washed quickly, and then differentiated by 1% acid alcohol for 30 seconds then washed for 10 minutes.
For counterstaining, slides were stained with eosin (Merck Millipore). For Sirius Red staining, liver sections were deparaffinized in xylene and hydrated in deionized (DI) water, and then stained with Picrosirius Red Stain Kit (Polysciences Asia-Pacific, Inc.), according to the protocol of the manufacturer.
Statistical Analysis
Data analysis was conducted using GraphPad Prism 6 (Graphpad Software, La Jolla, CA, USA). Statistical significance was set at p<0.05.
Results
Characteristic of ADSCs
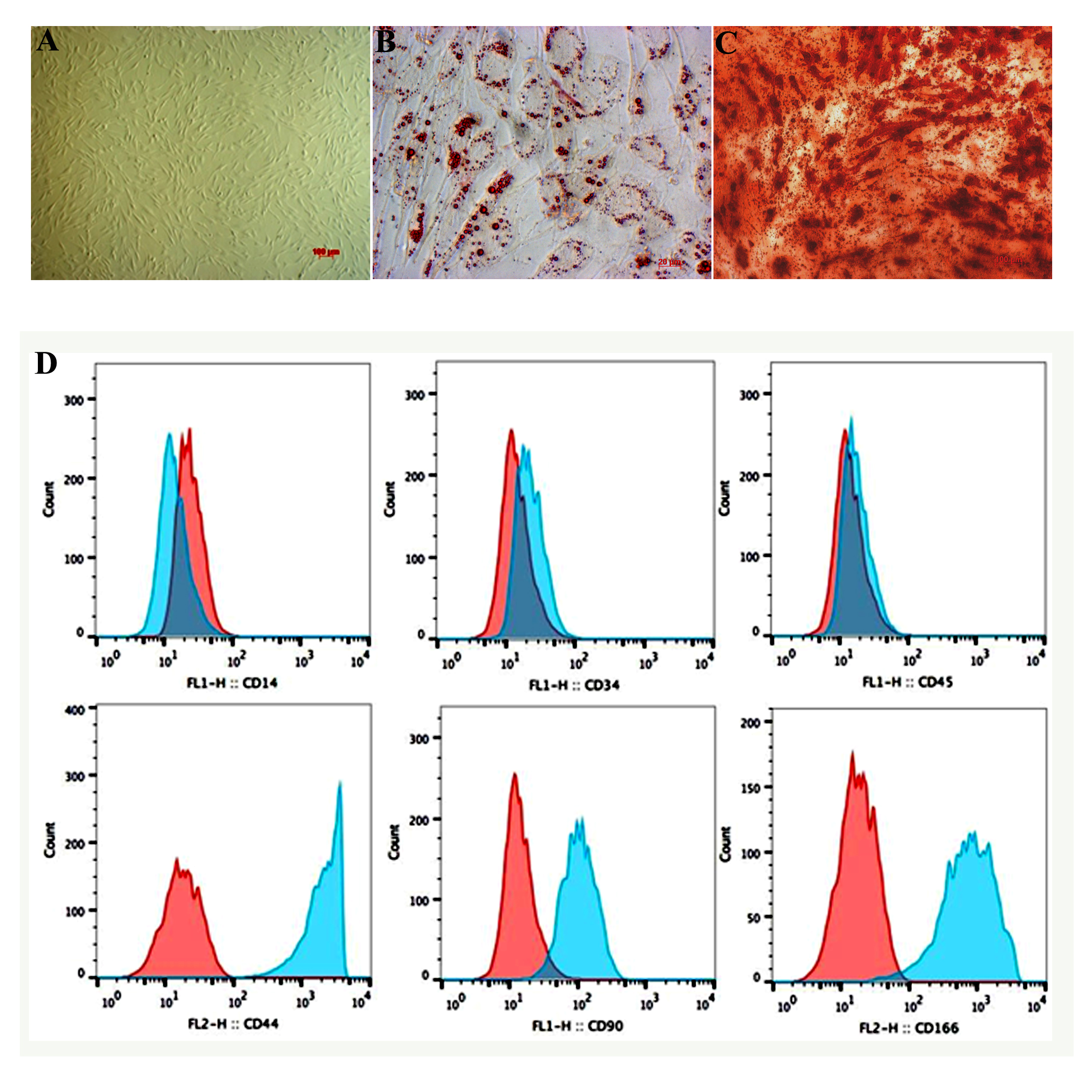
Third-passage ADSCs were defrosted and cultured in DMEM/F12 10% FBS and 1% antibiotics- antimycotics. Forty-eight hours after defrost, cells began to adhere to the surface of the plastic culture wells, and had the distinct appearance (long, fibroblast-like shape) of MSCs ( Figure 2A ).Forty-eight hours after thawing, ADSCs were harvested and validated for certain surface markers using flow cytometry. The cultured cells were CD44+, CD90+, and CD166+, and CD14-, CD34-, and CD45- ( Figure 2D ).
Differentiation into osteocytes and adipocytes
Twenty-one days after differentiation, cultured ADSCs transformed from the initial, distinct appearance into round-shaped cells, accumulating red-stained lipids with Oil red staining ( Figure 2B ). Similarly, ADSCs became positive with Alizarin red staining after 21 days of induction ( Figure 2D ).
HGF and PRP enhance cell proliferation
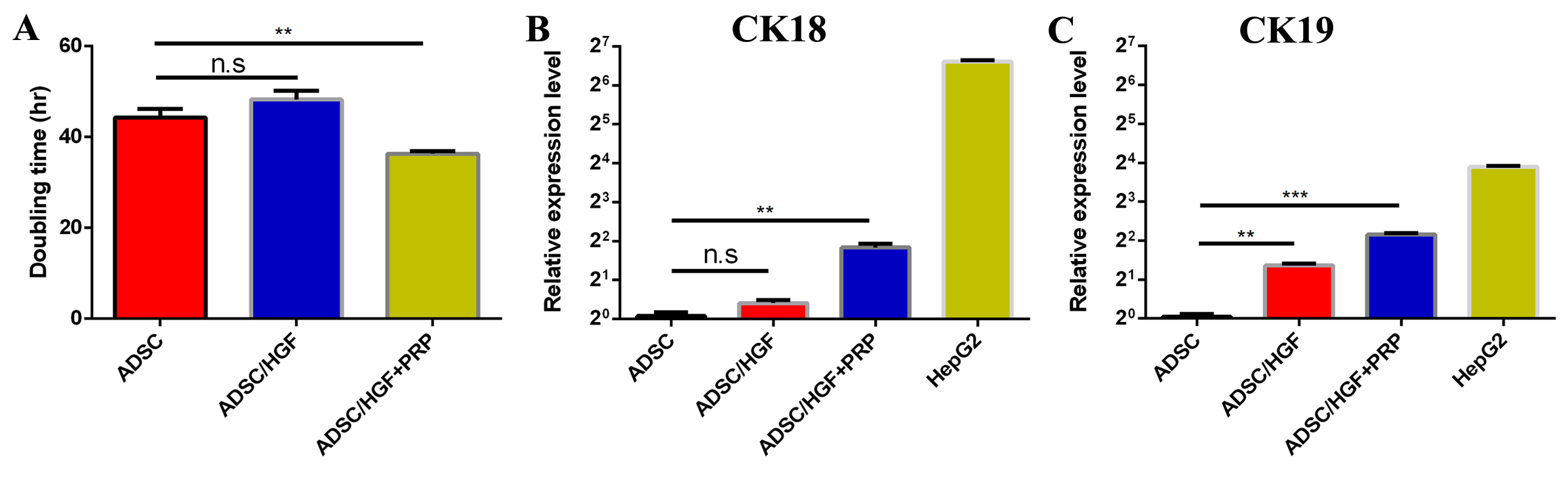
Doubling-time analyses amongst groups indicate that cells cultured in medium supplemented with HGF had the most expanded doubling-time (48.287±1.954 h). Cells cultured in medium supplemented with both HGF and PRP were the quickest to proliferate, with the shortest doubling time (36.339±0.559 h). All differences amongst groups were statistically significant (p<0.05) ( Figure 3A ).
HGF and PRP alter the expression of CK18 and CK19 genes
After seven days, expression of CK18 and CK19 was analyzed at the transcriptional level. In HGF treated group, gene expression of both Ck18 and Ck19 were significantly elevated compared to control (DMEM/F12 10% FBS) ( Figure 3B-C ). The increase of Ck18 and Ck19 gene expression in the ADSCs/HGF+PRP-treated group was each significantly different compared to ADSCs/DMEM F12 10% FBS-treated group.
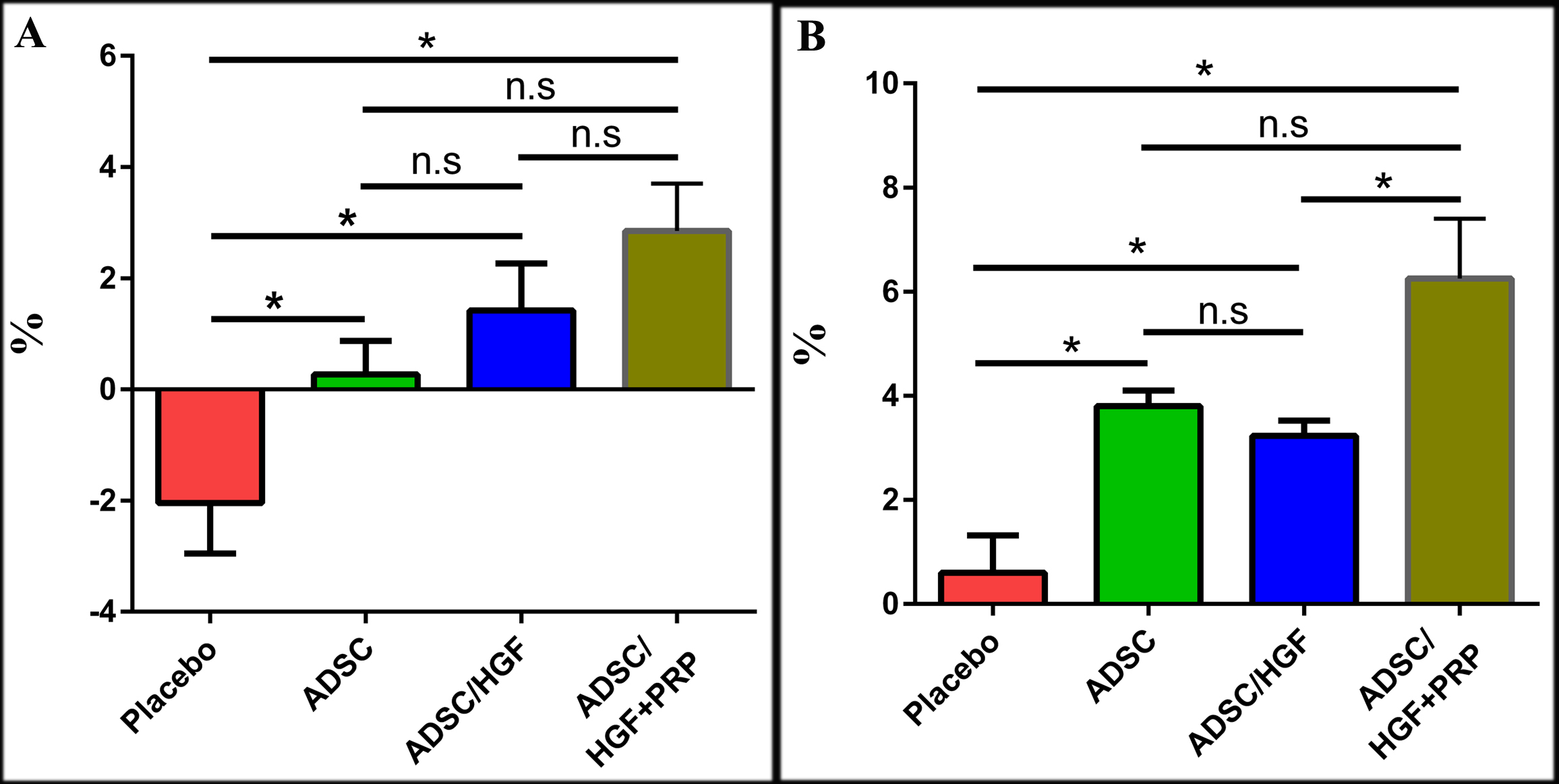
Transplantation of ADSCs pretreated with HGF and PRP improve weight loss in liver cirrhosis mouse model
There was a systemic weight loss in all mice treated with CCl4. The mean weight of CCl4-induced mice decreased by 12% (p<0.05) as compared with vehicle control (olive oil treatment). This result might suggest an effect of CCl4 on body weight; hence, the body weight was recorded as an initial criterion for evaluating the general physiology of the mice before and after receiving treatment Figure 4 .
After seven days of transplantation, the placebo group did not improve in body weight. In fact, body weight decreased by 2.04±0.91% as compared to that before commencing the treatment. Meanwhile, ADSC-treated group showed an enhancement of body weight, increasing by 0.27±0.61%. Moreover, ADSCs/HGF-treated and ADSCs/HGF+PRP-treated groups had increases in body weight of about 1.42 ± 0.85% and 2.85 ±0.85%, respectively ( Figure 4A ). After 14 days, the body weight of cell-treated mice was significantly improved. The body weight of ADSCs-treated group, ADSCs/HGF-treated group, and ADSCs/HGF+PRP-treated group were increased by 3.00±0.80%, 3.23±0.29%, and 6.25±1.15%, respectively, which were significant differently compared with the placebo (0.60±0.70%). In general, the cell-treated groups showed improvement in body weight as compared with the placebo; notably, the increase of body weight of mice treated with ADSCs/HGF+PRP was significantly higher compared to the other groups.
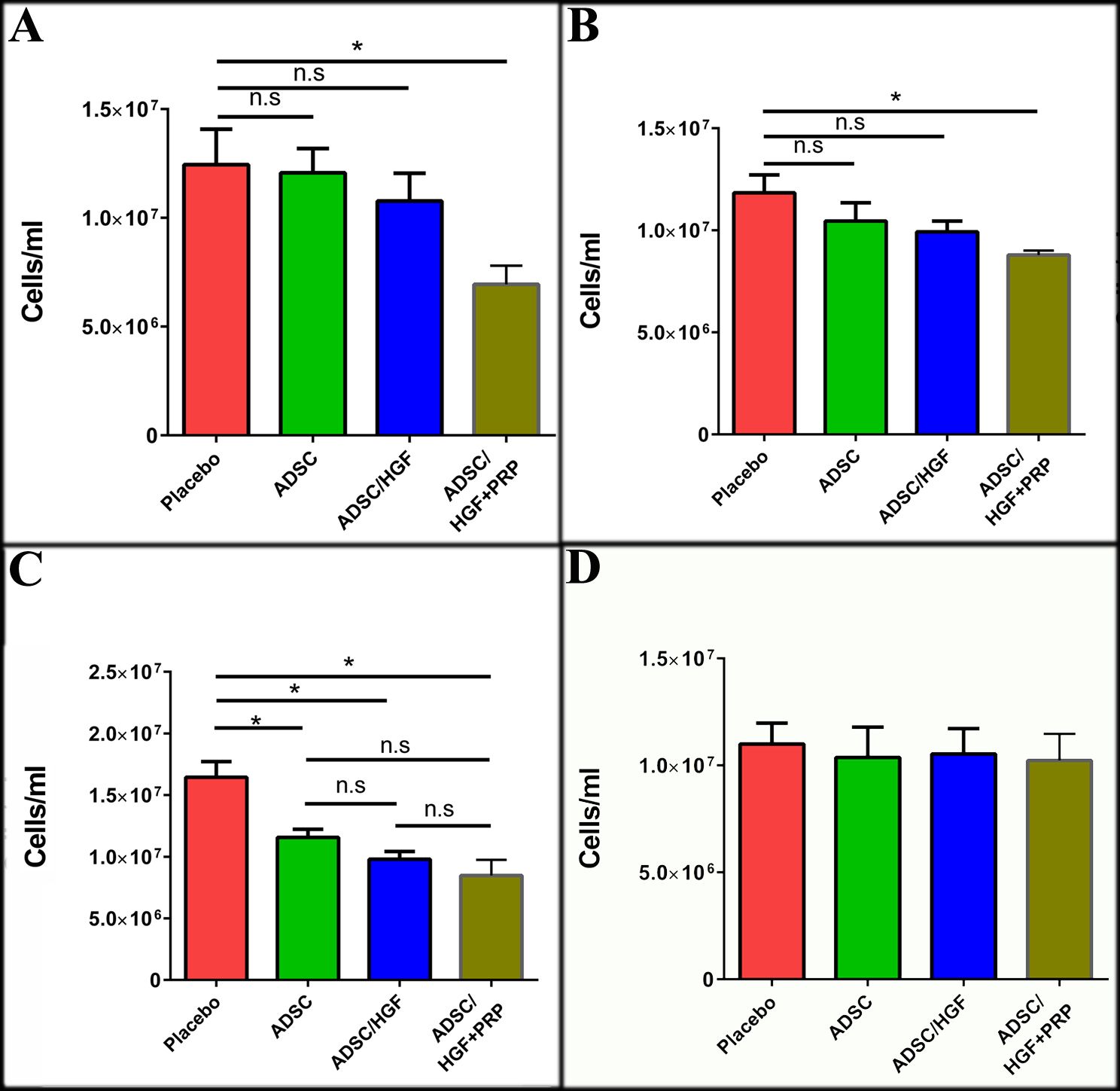
ADSCs cultured in medium supplemented with HGF and PRP improve hyper-leukocytosis in liver-damaged mice
The leukocyte count of the experimental mice was conducted on days 1, 3, 7 and 14 after cell transplantation. The total leukocyte count tended to drop down at 1-day post-transplantation in the 3 groups transplanted with cells as compared with the placebo (p<0.05). Importantly, ADSCs/HGF+PRP-treated group had the most decreased leukocyte count among the groups (p<0.05).
By days 3 and 7, all three ADSC-transplanted groups had a lower leukocyte count as compared with the placebo. By day 14, after cell transplantation, the leukocyte count of 3 cell-transplanted groups were not significantly different from each other Figure 5 .
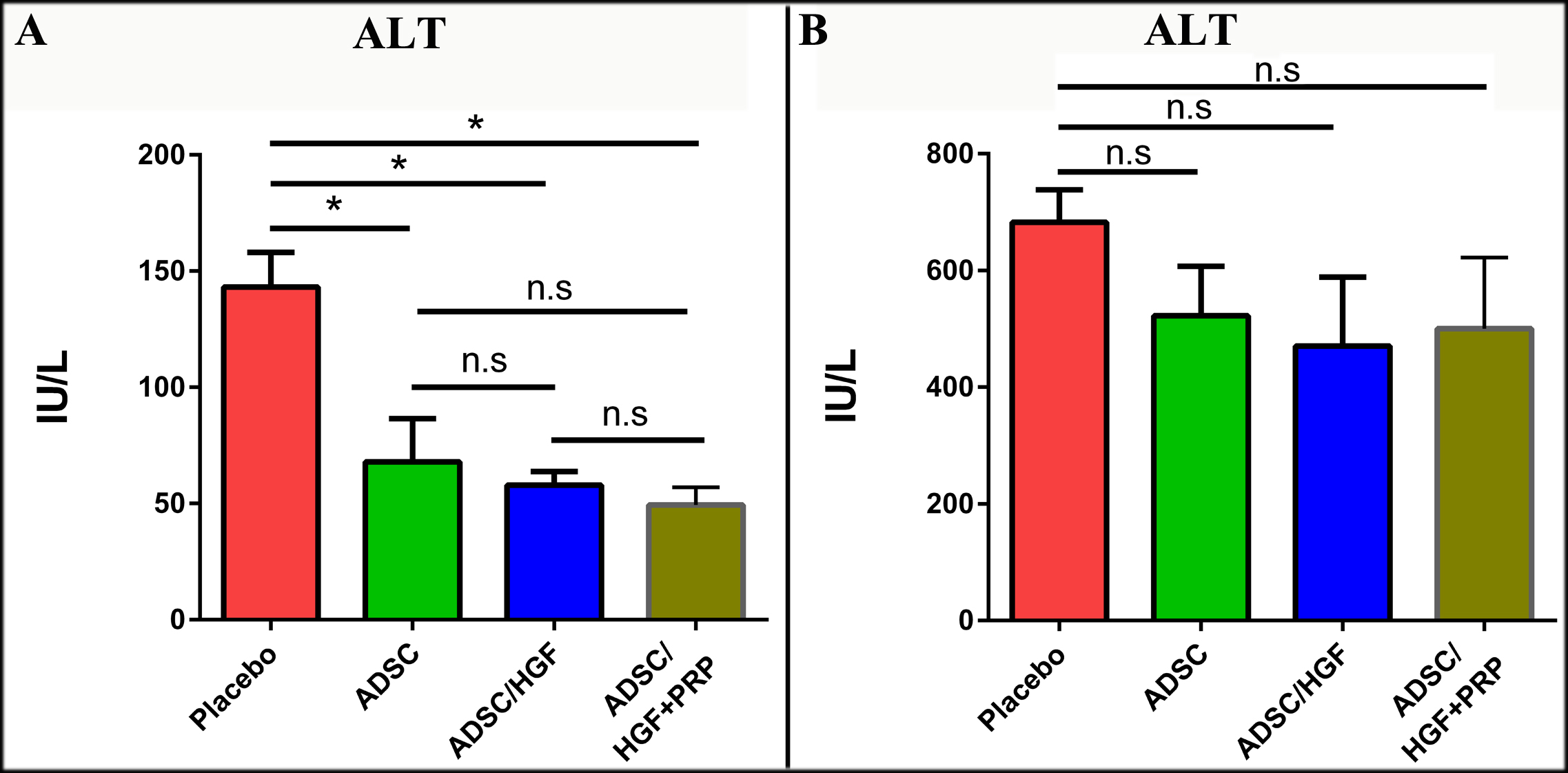
Transplantation of ADSCs cultured in media supplemented with HGF and PRP improve biochemistry indexes in a liver cirrhosis mouse model
After 7 days of transplantation, the ALT index in the placebo group (143.10±14.96 IU/L) was as equivalent as that in the CCl4-treated mouse model (144.00±10.85 IU/L) Figure 6 . On the other hand, all three groups transplanted with ADSCs had ALT index notably reduced; the ALX index was 67.9418.57 IU/L, 57.935.75 IU/L and 49.44±7.56 IU/L for the ADSCs-, ADSCs/HGF- and ADSCs/HGF+PRP-treated groups, respectively. After 14 days, ALT index was significantly elevated in all 4 experiment groups. Interestingly, the ALT level in the placebo group reached the highest point (682.6±55.55 IU/L). There was no statistical difference among the ADSCs-treated group (482.00±63.49 IU/L), ADSCs/HGF-treated group (470.60±118.20 IU/L), and ADSC/HGF+PRP-treated group (500.20±155.20 IU/L). These results indicate that the cell therapies used in this study could not significantly maintain their protective effect against progressing necrotic hepatocytes after 14 days of treatment.
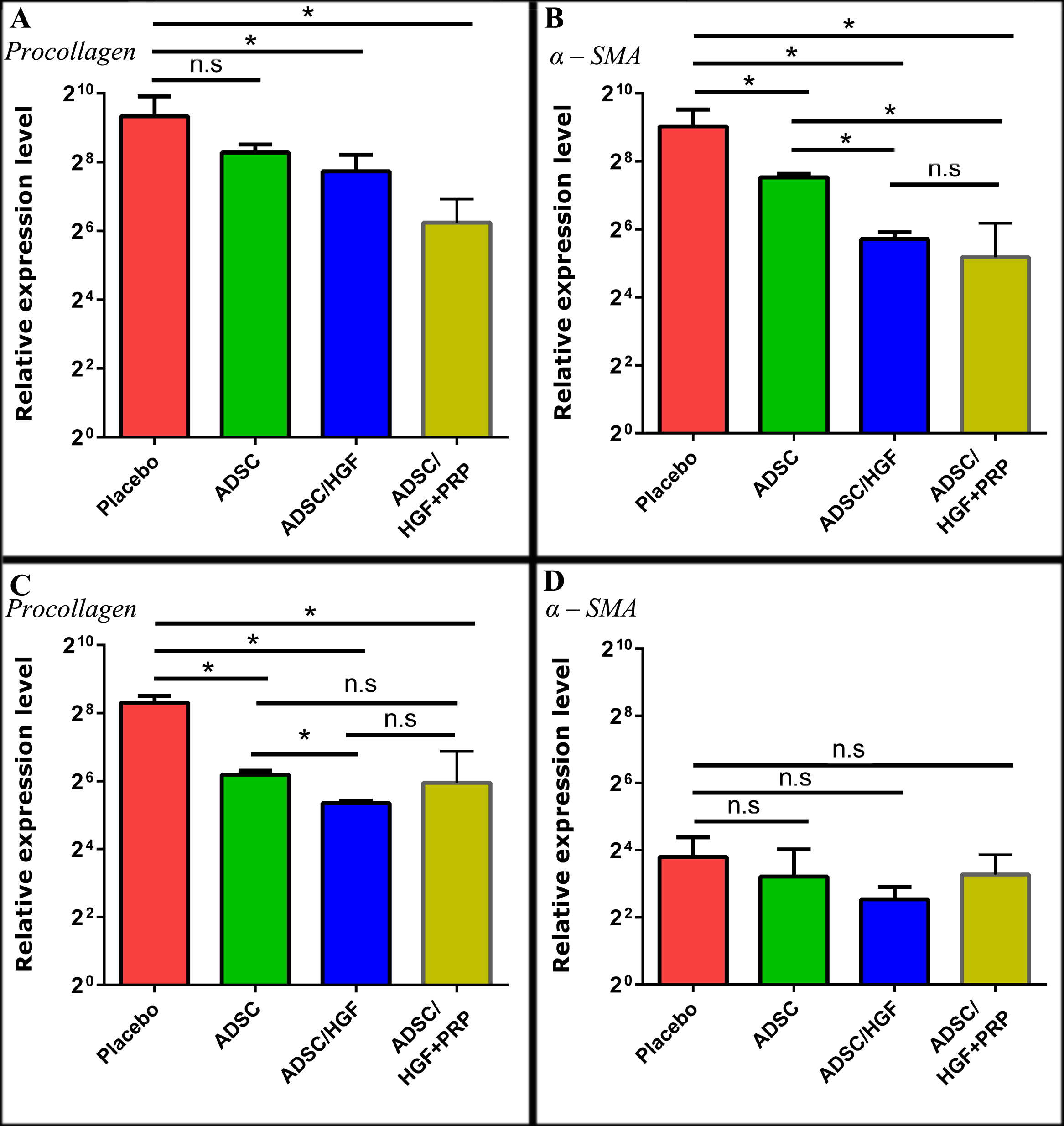
ADSCs cultured with HGF and PRP improve fibrosis-related gene- expression in mice
At day 7 post-transplantation, the gene-expression of procollagen-α1 had increased from 645.30
± 320.10 to 572.6 ± 291.5 in the placebo group. On the contrary, the reduction of procollagen- α1 expression was observed for all cell treatment groups. The procollagen-α1 expression of ADSCs/HGF+PRP group (75.64 ± 45.89) was lower than placebo group by 8.53-fold. Compared to ADSCs (310.50 ± 55.36), and ADSCs/HGF ((212.8 ± 84.35) treatment groups, the procollagen- α1 expression in ADSCs/HGF+PRP group was also significantly improved (lower) (p<0.05).
These results show that stem cell transplantation contributed to the downregulation of alpha- smooth muscle actin (α-SMA) gene expression. There was a significant difference (p<0.05) in α-SMA gene-expression between the placebo versus each of the cell treatment group. Notably, ADSCs pretreated with HGF and PRP showed a greater decrease in α-SMA gene expression (36.17 ± 36.09), compared to placebo, than ADSCs group (184.70 ± 14.06) or ADSCs/HGF group (52.41 ± 7.93) Figure 7 .
ADSCs cultured in medium supplemented with HGF and PRP reduce the formation of bridging fibrosis
After 7 days of treatment, ADSC transplantation significantly improved the overall histological structure as compared with placebo treatment. Notably, liver tissues of placebo-treated mice developed inflammation and numerous areas of fibrosis with necrotic cells ( Figure 8 ). In contrast, the conditions of the ADSC-treated groups were notably improved; there were fewer destructured zones and necrotic cells. There was no significance difference between the ADSC/DMEM F12 10% FBS-treated and ADSC/HGF-treated groups.
On day 14, the liver histology of all experiment groups seemed to be progressively affected during the examining period. Notably, bridging fibrosis emerged in the placebo group; however, the liver structure in the ADSCs-treated groups were intact.
Liver structures in the ADSC/DMEM F12 10% FBS-treated group still had fibrosis or inflammation bands in the proximity of the portal triad. There was no significant difference in transplanting ADSCs/HGF and ADSCs/HGF+PRP into the mice.
Discussion
Third-passage frozen ADSCs maintained the distinct characteristics of MSCs, including long- spreading, fibroblast-like shape. The ADSCs were also positive for markers CD44, CD90 and CD166, and negative for CD14, CD34 and CD45.
As well, they were capable of differentiating into osteocytes and adipocytes. Hence, after thawing, the ADSCs were suitable for further experiments. Adding either HGF or HGF+PRP to the culture medium altered several features of the cultured ADSCs. Firstly, the doubling time of cells cultured in the medium supplemented with 20 ng/ml HGF was elevated, as compared to the those cultured in DMEM F12 10% FBS. HGF plays an essential role in the differentiation of MSCs into hepatocytes, and this might be the cause for the increase in doubling time of cells cultured in HGF-supplemented media. The expression of CK18 and CK19, genes involved in the early process of hepatocyte differentiation, was evaluated after 7 days of treatment. The results revealed that treating cells with either HGF or HGF+PRP both enhanced the expression of the CK18 and CK19 genes significantly, as compared with that of cells in the DMEM F12 10% FBS. Culture medium. Specifically, the expression level in the cells receiving both HGF and PRP was approximately 3-fold higher than that for cells cultured in DMEM F12 10% FBS. Therefore, supplementing 20 ng/ml HGF and PRP as an alternative for FBS might have several additive effects on the cultured ADSCs. These might include maintaining the proliferation rate and orienting cells to differentiate into hepatocytes.
Regarding potential treatments for liver cirrhosis, recent studies have revealed that ADSC transplantation ameliorated pathological structures and functions of the liver.
Studies on ADSCs have indicated a variety of mechanisms in which they could contribute to relieving the disease conditions. Notably, some primary mechanisms of MSCS in fibrosis treatment are the migration of MSCs to the injured areas [38], MSC differentiation into functional hepatocyte-like cells in vitro and in vivo [39], and the capability of MSCs to exert immune modulation.
Moreover, in studies evaluating fusing and enhancing liver regeneration through secreting growth factors and/or fibrosis associated gene modulation [25][40], it was that shown that ADSC-based therapies in clinical applications could be efficacious and safe. Many studies have focused on signaling pathways (chemokines) and growth factors which could alter ADSC characteristics, including their mobility, proliferation, and stemness maintenance through enhancing the expression of gene-related chemokines and growth factors of ADSCs [13][38].
Research studies using HGF-overexpressing ADSCs have demonstrated liver regeneration and fibrosis reduction effects in mouse models [13]. Growth factors contained in PRP have been proven to elevate the healing and anti-inflammation processes [39]. PRP supplemented in culture medium was shown to accelerate the proliferation, mobility, and immune-modulating capability of hADSCs [2][4][8][27]. PRP has also been widely used as an alternative for FBS in various areas, such as cosmetics industry or osteoarthritis therapies [7][30].
In this study, HGF and PRP were co-added into the culture medium and the effect of HGF and PRP on liver cirrhosis was assessed both in vitro and in vivo. Mouse models of liver cirrhosis induced by CCl4 showed the characteristics of liver cirrhosis including hepatocyte necrosis, alterations in molecular signaling pathways, and formation of fibrotic structures. In this study, procedures for establishing the liver fibrosis model was adapted from previous publication by Nhung H. Truong et al. [31]. Oral administration of CCl4 to mice caused an elevation in ALT indexes and overexpression of fibrosis-associated genes (procollagen-α1, α-SMA, etc.), as well as the formation of fibrotic areas in the liver. These results are consistent with those in the study conducted by Nhung H. Truong et al [31].
The weight gain in mice receiving cell treatments (5.105 of hADSCs, or hADSCS/HGF, or hADSCs/HGF+PRP) showed initial recovery. It is noteworthy that the untreated mice tended to lose weight. Notably, weight gain indicates a positive effect of cell transplantation. Regarding liver injury, the ALT index is an indicator of the level of necrosis in the liver. In the CCl4- induced cirrhosis mouse model, free radicals cause cell membrane damage and lead to necrosis.
The consequence of hepatocyte necrosis is an elevation of ALT enzymes. At day 7 post- transplantation, there was a reduction of ALT level in all treated groups, which infers an alleviation of hepatocyte necrosis. ADSC transplantation could play several distinct roles in the inhibition of the oxidization caused by CCl4. In a study by Pan et al. (2015), human ADSCs were transplanted into a mouse model of non-alcoholic liver cirrhosis. The results showed that ADSCs efficiently reduced the lipid peroxidation process via elevating the expression of superoxide dismutase (SOD) and decreasing malondialdehyde (MDA) levels in the liver [25]. Many studies also indicate the anti-oxidization effect of human ADSC transplantation which is consistent with the reduction of ALT indexes in our study [41]-[43]. In this study, the total number of circulating leukocytes was considered as an indication for immune cell mobilization. By the 7th day after cell transplantation, the total number of leukocytes in the placebo group was significantly higher than in the ADSC groups. These results were consistent with the elevation of ALT activities in the placebo group. It was also demonstrated in the literature that ADSCs have an anti- inflammation effect via inhibition of CD4 T cells and macrophages [44]. Moreover, ADSCs could stimulate regulatory T cells (T-regs) and inhibit Th1, Th2 and Th17 cells, through regulation of inflammation-related cytokines (e.g. IL-4, IL-12, IL-17, TNF-α, IFN-γ, CD80, CD85, CD86, etc.) or immune-modulating cytokines (e.g. IL-10, TGF-β, Indolamine-2, 3-dioxygenase (IDO), etc.) [45]-[48]. Regarding fibrosis reduction, we evaluated gene expression (at the transcriptional level) of procollagen-α1 and α-SMA, which play essential roles in fibrosis formation. Fibrosis is characterized by an increase in the α-SMA positive cell population. Studies have revealed that activated-HSCs become myofibroblasts leading to a substantial collagen production [49]-[51]. This consequence could be explained by the over-expression of procollagen-1α and increase in the activity of enzymes which contribute to the transformation from procollagen into collagen, such as prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase, and collagen glucosyltransferase. In this study, overexpression of procollagen-1α and α-SMA at mRNA levels were observed in the CCl4 induced fibrosis mouse model, for the placebo treatment group. Interestingly, in the ADSCs-treated groups, there was a significant decrease in the expression procollagen-1α and α-SMA as compared to the placebo. These results are consistent with other studies which have shown that ADSCs possess an anti-fibrosis ability [52][53]. Due to secreting mechanisms, ADSCs could inhibit the proliferation as well as enhance the apoptosis of HSCs in vitro, and reduce the expression of procollagen-1α and α-SMA mRNA levels in liver fibrosis mice [53]. Notably, the down-regulation of procollagen-1α and α-SMA in ADSCs/HGF+PRP showed that HGF and PRP enhance anti-fibrosis ability of ADSCs. At the 14th day, there remained a low level gene expression of fibrosis-associated mRNA levels as compared with the placebo. Notably, the mRNA level of procollagen-1α in the groups treated with ADSCs was significantly lower than that of the placebo (p<0.05). This result indicates that ADSCs could sustain the preventative effect of collagen accumulation through reducing the expression of procollagen-1α and α-SMA after two weeks of treatment. Our results further provide evidence for positive effects of ADSCs in alleviating fibrosis condition in the liver.
Conclusion
In this study, ADSCs pre-treated with HGF and PRP for 7 days increased cell doubling time and enhanced expression of ck18 and ck19 genes. At 7-day post-transplantation, ADSCs pre-treated with HGF and PRP improved in body weight, total leukocytes, gene expression of pro-collagen and α-SMA, and in histological structure. At 14 days post-transplantation, there was an observed increase in the ALT enzyme and structure of collagen (caused by ongoing treatment of CCl4 at low dose). However, mice treated with ADSCs/HGF+PRP had a lower level of liver damage than the non-pretreated groups. Therefore, pre-treatment with PRP and HGF for 7 days enhanced the efficacy of ADSCs; these ADSCs were capable of alleviating liver fibrosis in vivo.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviation
ADSCs: Adipose-derived stem cells ALB: Albumin ALT: Alanine Amino Transferase AST: Aspartate Amino Transferase CK18: Cytokeratin 18 CK19: Cytokeratine 19 HGF: Hepatocyte Growth Factor MSCs: Mesenchymal Stem Cells PRP: Platelet Rich Plasma α-SMA: Smooth Muscle Actin alpha
Ethics approval and consent to participate
This study was accepted by the Ethical Committee of Stem Cell Institute, University of Science, VNU-HCMUS. The animal was housed according to the guideline of Laboratory of Animal Care and Use, Stem Cell Institute, University of Science, VNU-HCMUS.
Availability of data and materials
Will be provided if requested.
Competing interests
The authors declare that there is no conflict of interests.
Funding
The study was supported by Science and Technology Incubator Youth Program, managed by the Center for Science and Technology Development, Ho Chi Minh Communist Youth Union, the contract number is “07/2017/HÐ-KHCN- V”.
Authors’ contributions
Nam Nguyen and Trinh Le have contributed equally in conducting experiments, acquisition of data and compose the manuscript. Huy Q. Do, Thanh M. Dang, Yen KT Nguyen, and Ngoc H. Vo determined and analyzed the liver function and gene expression. Nhung Hai Truong made substantial contributions to conception and design, data analysis, interpretation of data and gave final approval of the manuscript to be submitted.
References
-
AA
Mokdad,
AD
Lopez,
S
Shahraz,
R
Lozano,
AH
Mokdad,
J
Stanaway,
CJ
Murray,
M
Naghavi.
Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC medicine.
2014;
12
:
145
.
View Article PubMed PMC Google Scholar -
A
Pascher,
M
Nebrig,
P
Neuhaus.
Irreversible liver failure: treatment by transplantation: part 3 of a series on liver cirrhosis. Dtsch Arztebl Int.
2013;
110
:
167-73
.
View Article Google Scholar -
Y
Wang,
F
Lian,
J
Li,
W
Fan,
H
Xu,
X
Yang,
L
Liang,
W
Chen,
J
Yang.
Adipose derived mesenchymal stem cells transplantation via portal vein improves microcirculation and ameliorates liver fibrosis induced by CCl4 in rats. J Transl Med.
2012;
10
:
133
.
View Article Google Scholar -
A
Seki,
Y
Sakai,
T
Komura,
A
Nasti,
K
Yoshida,
M
Higashimoto,
M
Honda,
S
Usui,
M
Takamura,
T
Takamura,
T
Ochiya,
K
Furuichi,
T
Wada,
S
Kaneko.
Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology.
2013;
58
:
1133-42
.
View Article PubMed Google Scholar -
Y
Zhang,
XM
Chen,
DL
Sun.
Effects of coencapsulation of hepatocytes with adipose- derived stem cells in the treatment of rats with acute-on-chronic liver failure. Int J Artif Organs.
2014;
37
:
133-41
.
View Article PubMed Google Scholar -
KA
Cho,
GW
Lim,
SY
Joo,
SY
Woo,
JY
Seoh,
SJ
Cho,
HS
Han,
KH
Ryu.
Transplantation of bone marrow cells reduces CCl4 -induced liver fibrosis in mice. Liver Int.
2011;
31
:
932-9
.
View Article PubMed Google Scholar -
XR
Ma,
YL
Tang,
M
Xuan,
Z
Chang,
XY
Wang,
XH
Liang.
Transplantation of autologous mesenchymal stem cells for end-stage liver cirrhosis: a meta-analysis based on seven controlled trials. Gastroenterol Res Pract.
2015;
2015
:
908275
.
View Article PubMed PMC Google Scholar -
MTA
Aziz,
MFE
Asmar,
S
Mostafa,
H
Salama,
HM
Atta,
S
Mahfouz,
NK
Roshdy,
LA
Rashed,
D
Sabry,
N
Hasan,
M
Mahmoud,
D
Elderwy.
Reversal of Hepatic Fibrosis by Human CD34(+) Stem/Progenitor Cell Transplantation in Rats. International Journal of Stem Cells.
2010;
3
:
161-174
.
View Article PubMed PMC Google Scholar -
F
Ishikawa,
CJ
Drake,
S
Yang,
P
Fleming,
H
Minamiguchi,
RP
Visconti,
CV
Crosby,
WS
Argraves,
M
Harada,
JK
L. L.,
AG
Livingston,
JR
Wingard,
M
Ogawa.
Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann N Y Acad Sci.
2003;
996
:
174-85
.
View Article PubMed Google Scholar -
M
Gazdic,
A
Arsenijevic,
BS
Markovic,
A
Volarevic,
I
Dimova,
V
Djonov,
N
Arsenijevic,
M
Stojkovic,
V
Volarevic.
Mesenchymal Stem Cell-Dependent Modulation of Liver Diseases. Int J Biol Sci.
2017;
13
:
1109-1117
.
View Article PubMed PMC Google Scholar -
DP
Kavanagh,
S
Suresh,
PN
Newsome,
J
Frampton,
N
Kalia.
Pretreatment of Mesenchymal Stem Cells Manipulates Their Vasculoprotective Potential While Not Altering Their Homing Within the Injured Gut. Stem Cells.
2015;
33
:
2785-97
.
View Article PubMed PMC Google Scholar -
A
Moroz,
RA
Bittencourt,
RP
Almeida,
SL
Felisbino,
E
Deffune.
Platelet lysate 3D scaffold supports mesenchymal stem cell chondrogenesis: an improved approach in cartilage tissue engineering. Platelets.
2013;
24
:
219-25
.
View Article Google Scholar -
L
Lai,
J
Chen,
X
Wei,
M
Huang,
X
Hu,
R
Yang,
X
Jiang,
H
Shan.
Transplantation of MSCs Overexpressing HGF into a Rat Model of Liver Fibrosis. Mol Imaging Biol.
2016;
18
:
43-51
.
View Article PubMed Google Scholar -
T
Nakamura,
K
Nawa,
A
Ichihara.
Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun.
1984;
122
:
1450-9
.
View Article Google Scholar -
T
Nakamura,
T
Nishizawa,
M
Hagiya,
T
Seki,
M
Shimonishi,
A
Sugimura,
K
Tashiro,
S
Shimizu.
Molecular cloning and expression of human hepatocyte growth factor. Nature.
1989;
342
:
440-3
.
View Article PubMed Google Scholar -
H
Funakoshi,
T
Nakamura.
Hepatocyte growth factor: from diagnosis to clinical applications. Clinica chimica acta.
2003;
327
:
1-23
.
View Article Google Scholar -
C
Schmidt,
F
Bladt,
S
Goedecke,
V
Brinkmann.
Scatter factor/hepatocyte growth factor is essential for liver development. Nature.
1995;
373
:
699
.
View Article PubMed Google Scholar -
N
Fausto.
Liver regeneration. Journal of hepatology.
2000;
32
:
19-31
.
View Article Google Scholar -
DF
Balkovetz.
Evidence that hepatocyte growth factor abrogates contact inhibition of mitosis in Madin-Darby canine kidney cell monolayers. Life sciences.
1999;
64
:
1393-1401
.
View Article Google Scholar -
J
Kamp,
V
Paefgen,
M
Woltje,
M
Bobel,
J
Jaekel,
B
Rath,
N
Labude,
R
Knuchel,
W
Jahnen- Dechent,
S
Neuss.
Mesenchymal stem cells can be recruited to wounded tissue via hepatocyte growth factor-loaded biomaterials. J Tissue Eng Regen Med.
2017;
11
:
2988-2998
.
View Article PubMed Google Scholar -
G
Forte,
M
Minieri,
P
Cossa,
D
Antenucci,
M
Sala,
V
Gnocchi,
R
Fiaccavento,
F
Carotenuto,
PD
Vito,
PM
Baldini.
Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem cells.
2006;
24
:
23-33
.
View Article Google Scholar -
S
Neuss,
E
Becher,
M
Wöltje,
L
Tietze,
W
Jahnen-Dechent.
Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem cells.
2004;
22
:
405-414
.
View Article Google Scholar -
S
Valente,
C
Ciavarella,
E
Pasanisi,
F
Ricci,
A
Stella,
G
Pasquinelli.
Hepatocyte growth factor effects on mesenchymal stem cells derived from human arteries: a novel strategy to accelerate vascular ulcer wound healing. Stem cells international 2016.
2015
.
-
TE
Foster,
BL
Puskas,
BR
Mandelbaum,
MB
Gerhardt,
SA
Rodeo.
Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med.
2009;
37
:
2259-72
.
View Article Google Scholar -
BL
Eppley,
JE
Woodell,
J
Higgins.
Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plastic and reconstructive surgery.
2004;
114
:
1502-1508
.
View Article PubMed Google Scholar -
PR
Amable,
RBV
Carias,
MVT
Teixeira,
Pacheco
Ítalo da Cruz,
RJFC
do Amaral,
JM
Granjeiro,
R
Borojevic.
Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem cell research & therapy.
2013;
4
:
67
.
View Article PubMed PMC Google Scholar -
K
Bieback.
Platelet Lysate as Replacement for Fetal Bovine Serum in Mesenchymal Stromal Cell Cultures. Transfusion Medicine and Hemotherapy.
2013;
40
:
326-335
.
View Article PubMed PMC Google Scholar -
C
Doucet,
I
Ernou,
Y
Zhang,
JR
Llense,
L
Begot,
X
Holy,
JJ
Lataillade.
Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol.
2005;
205
:
228-36
.
View Article Google Scholar -
PR
Amable,
MVT
Teixeira,
RBV
Carias,
JM
Granjeiro,
R
Borojevic.
Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PloS one.
2014;
9
:
e104662
.
View Article PubMed PMC Google Scholar -
E
Rubio-Azpeitia,
I
Andia.
Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles, ligaments and tendons journal.
2014;
4
:
52
.
View Article Google Scholar -
NH
Truong,
NH
Nguyen,
NTK
Nguyen,
HM
Le,
GH
Tran,
N
Huynh,
TV
Nguyen.
Establishment of a standardized mouse model of hepatic fibrosis for biomedical research. Biomedical Research and Therapy.
2014;
1
:
43-49
.
View Article Google Scholar -
PK
Ngoc,
PV
Phuc,
TH
Nhung,
DT
Thuy,
NTM
Nguyet.
Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells. Human cell.
2011;
24
:
86-95
.
View Article PubMed Google Scholar -
PV
Pham,
PTM
Nguyen,
ATQ
Nguyen,
VM
Pham,
ANT
Bui,
LTT
Dang,
KG
Nguyen,
NK
Phan.
Improved differentiation of umbilical cord blood-derived mesenchymal stem cells into insulin-producing cells by PDX-1 mRNA transfection. Differentiation.
2014;
87
:
200-208
.
View Article PubMed Google Scholar -
al
Dan NTM et.
Evaluation of effects of Lingzhi mushroom (Ganoderma lucidum) on neural stem cells isolated from embryonic mouse brain (Mus musculus var. albino). Asia-Pacific Journal of Science and Technology.
2017;
19
:
181-189
.
-
AM
Sousa,
T
Liu,
O
Guevara,
J
Stevens,
BL
Fanburg,
M
Gaestel,
D
Toksoz,
US
Kayyali.
Smooth muscle a-actin expression and myofibroblast differentiation by TGF ß are dependent upon MK2. Journal of cellular biochemistry.
2007;
100
:
1581-1592
.
View Article PubMed PMC Google Scholar -
K
Sa-ngiamsuntorn,
A
Wongkajornsilp,
K
Kasetsinsombat,
S
Duangsa-ard,
L
Nuntakarn,
S
Borwornpinyo,
P
Akarasereenont,
S
Limsrichamrern,
S
Hongeng.
Upregulation of CYP 450s expression of immortalized hepatocyte-like cells derived from mesenchymal stem cells by enzyme inducers. BMC biotechnology.
2011;
11
:
89
.
View Article PubMed PMC Google Scholar -
RM
Baertschiger,
V
Serre-Beinier,
P
Morel,
D
Bosco,
M
Peyrou,
S
Clément,
A
Sgroi,
A
Kaelin,
LH
Buhler,
C
Gonelle-Gispert.
Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PloS one.
2009;
4
:
e6657
.
View Article PubMed Google Scholar -
Z
Zhang,
FS
Wang.
Stem cell therapies for liver failure and cirrhosis. Journal of Hepatology.
2013;
59
:
183-185
.
View Article PubMed Google Scholar -
M
Sgodda,
H
Aurich,
S
Kleist,
I
Aurich,
S
König,
MM
Dollinger,
WE
Fleig,
B
Christ.
Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Experimental cell research.
2007;
313
:
2875-2886
.
View Article PubMed Google Scholar -
RP
Meier,
YD
Müller,
P
Morel,
C
Gonelle-Gispert,
LH
Bühler.
Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence?. Stem cell research.
2013;
11
:
1348-1364
.
View Article PubMed Google Scholar -
S
Zhang,
Z
Dong,
Z
Peng,
F
Lu.
Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by D-galactose. PLoS One.
2014;
9
:
e97573
.
View Article PubMed PMC Google Scholar -
WS
Kim,
BS
Park,
JH
Sung.
The wound-healing and antioxidant effects of adipose- derived stem cells. Expert opinion on biological therapy.
2009;
9
:
879-887
.
View Article PubMed Google Scholar -
YB
Chae,
JS
Lee,
HJ
Park,
IH
Park,
MM
Kim,
YH
Park,
DS
Kim,
JH
Lee.
Advanced adipose-derived stem cell protein extracts with antioxidant activity modulates matrix metalloproteinases in human dermal fibroblasts. environmental toxicology and pharmacology.
2012;
34
:
263-271
.
-
M
Higashimoto,
Y
Sakai,
M
Takamura,
S
Usui,
A
Nasti,
K
Yoshida,
A
Seki,
T
Komura,
M
Honda,
al
Wada T et.
Adipose tissue derived stromal stem cell therapy in murine ConA- derived hepatitis is dependent on myeloid-lineage and CD4+ T-cell suppression. European journal of immunology.
2013;
43
:
2956-2968
.
View Article PubMed Google Scholar -
A
Mohammadzadeh,
AA
Pourfathollah,
S
Shahrokhi,
SM
Hashemi,
SLA
Moradi,
M
Soleimani.
Immunomodulatory effects of adipose-derived mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. International immunopharmacology.
2014;
20
:
316-321
.
View Article Google Scholar -
L
Frese,
PE
Dijkman,
SP
Hoerstrup.
Adipose tissue-derived stem cells in regenerative medicine. Transfusion Medicine and Hemotherapy.
2016;
43
:
268-274
.
View Article PubMed PMC Google Scholar -
F
Cao,
T
Liu,
Y
Xu,
D
Xu,
S
Feng.
Culture and properties of adipose-derived mesenchymal stem cells: characteristics in vitro and immunosuppression in vivo. International journal of clinical and experimental pathology.
2015;
8
:
7694
.
PubMed PMC Google Scholar -
RP
Meier,
YD
Müller,
P
Morel,
C
Gonelle-Gispert,
LH
Bühler.
Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence?. Stem cell research.
2013;
11
:
1348-1364
.
View Article PubMed Google Scholar -
SL
Friedman.
Mechanisms of hepatic fibrogenesis. Gastroenterology.
2008;
134
:
1655-1669
.
View Article PubMed PMC Google Scholar -
D
Li,
S
Friedman.
Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. Journal of gastroenterology and hepatology.
1999;
14
:
618-633
.
View Article PubMed Google Scholar -
R
Safadi,
SL
Friedman.
Hepatic fibrosis-role of hepatic stellate cell activation. MedGenMed: Medscape general medicine.
2002;
4
:
27-27
.
PubMed Google Scholar -
HJ
Harn,
SZ
Lin,
SH
Hung,
YM
Subeq,
YS
Li,
WS
Syu,
DC
Ding,
RP
Lee,
DK
Hsieh,
al
Lin PC et.
Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell transplantation.
2012;
21
:
2753-2764
.
View Article PubMed Google Scholar -
F
Yu,
S
Ji,
L
Su,
L
Wan,
S
Zhang,
C
Dai,
Y
Wang,
J
Fu,
Q
Zhang.
Adipose-derived mesenchymal stem cells inhibit activation of hepatic stellate cells in vitro and ameliorate rat liver fibrosis in vivo. Journal of the Formosan Medical Association.
2015;
114
:
130-138
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 5 (2018)
Page No.: 2332-2348
Published on: 2018-05-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7730 times
- Download PDF downloaded - 1608 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress