Abstract
Introduction: There is evidence of the curing effects of prebiotics in promoting growth of bifid bacterium in the digestive system and the central role of bacteria colonization in the pathogenesis of ventilator-associated pneumonia (VAP). The purpose of this study was to evaluate the effects of administration of a prebiotic, namely fenugreek seeds, on VAP prevention and clinical outcomes in critically ill patients.
Methods: In this study, 60 mechanically ventilated patients were randomly divided into 2 groups (n=30 per group). Group 1 was given fenugreek seed powder by gavage, twice a day in addition to routine care, while group 2 received only routine care. Demographic and clinical data were recorded and clinical responses to the primary component (prevalence of VAP) and secondary component (other clinical factors) were interpreted. Data were analyzed via SPSS v.20, using student t-test, chi-square test, repeated measure ANOVA, and Wilcoxon test.
Result: There was a significant reduction of patients diagnosed with VAP, as well as clostridium difficileassociated diarrhea and some complications of mechanical ventilation, in group 1 when compared to group 2. In addition, improvement in VAP was significantly greater for group 1 as compared with group 2. Mortality rates were not different between the two groups.
Conclusion: The present study demonstrated that daily diet with fenugreek seeds can be used as an add-on therapy with other medications in prevention of VAP. As a result, the use of fenugreek seeds in the treatment plan of patients undergoing long-term intubation is recommended.
Background
Ventilator-associated pneumonia (VAP) is still a hazardous complication which is common during ventilation [1]. Indeed, VAP is pneumonia which develops within 48 hours of mechanical ventilation by means of an endotracheal tube or tracheostomy. VAP may be responsible for approximately 27–47% of healthcare-associated infections. It increases mortality risk, severe morbidity, prolonged hospital stays, and additional hospital costs [2].
Ample research has established that gut flora balance has an important role in reducing infectious complications and multiple organ dysfunction syndrome [3]. The main pathogenesis of VAP consists of colonization of the upper aerodigestive tract with pathogenic bacteria and microaspiration of infected oropharyngeal secretions around the endotracheal tube cuff into the lung [4]–[9]. Evidence suggests that prebiotics like probiotics can reduce oropharyngeal and gastric colonization and can modulate immune cell function [2][4]. Critical illness is characterized by excessive growth of pathogenic bacteria and lack of commensal bacteria, which finally disrupt immunological functions [5]. William Manzanares et al. searched databases from 1980 to 2016 and found 13 randomized controlled trials (RCTs) involving 1,969 patients. They found that probiotic and symbiotic therapy remarkably reduced endogenous flora in the gut, which could be correlated with the occurrence of VAP [5].
Probiotic, prebiotic and symbiotic treatments support the maintenance and reparation of gut microbiota and gut environment. Probiotics introduce good bacteria into the gut; however, they should be used with caution and require compliance. Probiotics may be ineffective before persisting in the lower digestive tract [6][7]. Prebiotics are defined as a selectively fermented, non- digestible ingredients that specifically stimulate viability of some groups of beneficial bacteria present in any individual’s gut, especially those that produce short chain fatty acids [4]. Most importantly, administration of probiotics can carry the inherent risk of iatrogenic infection to critically ill patients [8][9]. Science has proven that using prebiotics can improve overall health of the host; for example, prebiotics can increase bone density, strengthen the immune system, lead to better control of weight and appetite, improve bowel regularity, and improve mental health [10]–[14].
Fenugreek seeds (Trigonella foenum-graecum) contain 45-60% carbohydrates, mainly mucilaginous fiber (galactomannans) that act as prebiotics [10]. Fenugreek seed can enhance innate immunity through multiple mechanisms, including reinforcement of the intestinal barrier function, rectification of the gut flora by induction of antimicrobial peptides in cell, and facilitating epithelial connection [10]–[12],[15]–[17].
Tin an end-stage renal disease (ESRD) study, conducted by the National Institute of Diabetes and Digestive and Kidney diseases (NIDDK), hemodialysis novel therapies (HDNT) were studied, that based on Prebiotin prebiotic fiber (p-inulin). It was found that the prebiotic altered the composition and function of the gut flora and, ultimately, improved and reduced systemic inflammation in chronic kidney disease (CKD) patients [13]. Moreover, Rehab et al. (2015) evaluated the antimicrobial activities of fenugreek seeds against gram-negative and gram- positive bacteria, as well as other microorganisms, using two different methods: agar disc diffusion and agar-well diffusion methods. The results indicated that boiling water extract contains antimicrobial activity against gram-negative and gram-positive bacteria, and other microorganisms, such as Escherichia coli (E. coli), Proteus vulgaris (P. vulgaris), Staphylococcus aureus (St. aureus), Candida albicans (C. albicans), Staphylococcus epidermis (St. epidermis), and Staphylococcus saprophyticus [14]. The clinical significance of the prebiotics are less known. Therefore, in this study, we aim to determine the effects of fenugreek seed powder when used as add-on therapy with other drugs in the prevention of VAP in patients receiving mechanical ventilation.
Methods
The clinical trial group led two parallel randomized studies. The Institutional Review Board of Sabzevar University approved the protocol of the present study (Code of ethics: IR.MEDSAB.REC.1394.128). Patients were recruited from April 2015 to June 2016 at two intensive care unit (ICU) centers (Dr, Shahid Beheshti’s unit and the Mohammad Vasei Hospitals, Sabzevar, Iran).
Inclusion criteria were as follows
- Adult patients at least 20 years of age
- Mechanical ventilated with an endotracheal tube for more than 48 h
- Enteral nutrition order with nasogastric tube
- No pregnancy
Exit criteria during the study included:
- Not satisfied to continue study
- Extubation or placement of a tracheostomy
- Intestinal injury during the current admission
- Bleeding disorders or having internalized normalized ratio (INR)2
- Unable to administer the first dose of fenugreek seed powder drug within 24 hours of intubation
- Transfer to another hospital.
Study design
Each patient who met the inclusion criteria and completed informed consents was enrolled; all risks and benefits were explained by the lead investigator. Then each patient (sampler) was randomly assigned to one of two groups (group 1 or group 2) and the same patient was in the opposite group. Sampling was continued until completion of the sample size; group 1 and group 2 consisted of 30 patients each.
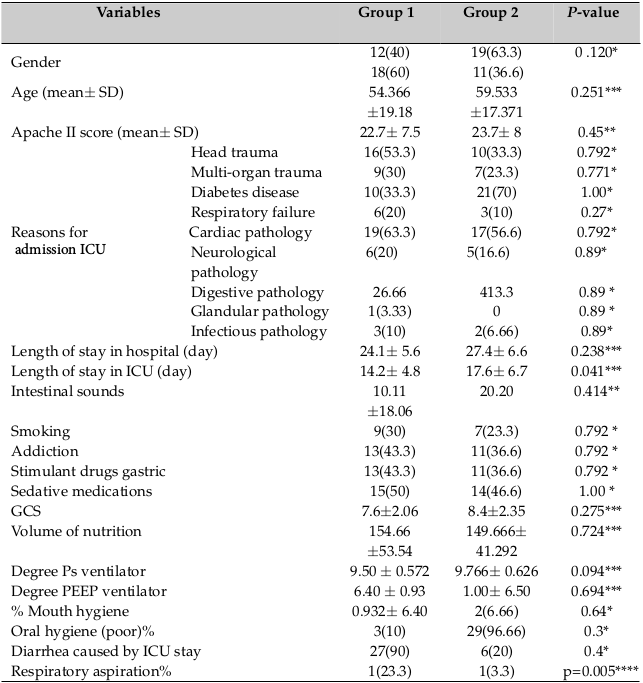
Demographic information and baseline clinical data (clinical profile, medical history, APACHE II score, and gastric residual volumes) were collected on the first day of admission. Patients were equally divided into 2 groups ( Table 1 ). Patients participated in the study until vents, such as extubation, tracheotomy, placement, discharge, and/or death, occurred. Patients received all VAP-preventive measures throughout the study. The protocol remained unchanged during the study. The nurses, doctors, students, and other personnel were blinded from the study. Group 1 received 3 g of fenugreek seed powder twice a day via a nasogastric tube; Group 2 received only the routine care.
The first gavage fenugreek seed was given within 24 hours of intubation. To achieve a double- blinded trial, a code was given to all the sample dishes. Residual gastric volume was measured before each gavage. Patients of the two groups were alternately fed using a 60-cc syringe without a piston within 10-15 minutes, and at an altitude of 12 inches above the patient’s stomach every 3 h. During lavage and gavage, the patient’s head was elevated 35º from the bed; this position was maintained until one hour after. After pouring warm soup into the gavage dishes, the lead investigator added 3 g of fenugreek seed powder into the sample dishes (of group 1. At the 12th 18th hour, adding fenugreek seeds did not change the color or appearance of the soup.
VAP was diagnosed based on the clinical scores, according to the American College of Chest Physicians (ACCP). Clinical scores reflect the new and persistent infiltrates on chest radiographs. The infiltrates were associated to two or three supporting factors: fever (38.5◦C or 35.0◦C), leukocytosis (white blood cells 10,000 mm3 or 3,000 mm3), and purulent sputum [8].
Respiratory aspiration was detected in the presence of a biological fluid or any trace of the contents of the gavage, or particulate matter, in the suctioned secretions of airways and bronchoscope [18][19].
The reasons for using fenugreek seed in this study was that, in small amounts, it can change the flora. Moreover, it is a native plant that is physiologically compatible with the body of indigenous people of this region. Besides, fenugreek is a rich source of calcium, iron, vitamins A, B1, C, nicotinic acid, alkaloids, riboflavin, carotene, thiamine, niacin, free amino acids, and proteins. It is also effective in digestion and the treatment of hypercholesterolemia and hyperglycemia [20]–[22]. Evaluating the incidence of VAP was the primary aim of this study. The secondary aims include evaluating the following: frequent development of respiratory aspiration, mortality rate, length of onset of ventilation to the incidence of VAP, duration of mechanical ventilation, length of stay in hospital, length of stay in ICU, gastric residual volumes, Clostridium difficile-associated diarrhea (three or more loose stools per 24 h period), other ICU-associated diarrhea (presumably because of acute disorder, antibiotic administration and dietary changes), constipation (not defecation) for 3 consecutive days, clinical status of patients, and hospital charges.
The clinical status of patients was defined as follows:
1) Complete recovery: fever ending after 48 hours, resolving the early findings of a physical examination of the lung after 1 week, recovery of leukocytosis after 4 days, and recovery of CXR in 4 to 12 weeks.
2) Partial recovery: fever ending after 4-7 days and improvement of physical examinations for more than 10 days.
3) Lack of recovery: not resolving the symptoms or incidence of complications. 4- Death at the time of hospitalization in ICU or in other parts of the hospital.
Results
A total of 116 patients were screened from the aspect of initial entrance and exclusion criteria of the study. Of the 60 patients (100%) that were randomly selected, 18 patients (30%) were excluded during the study. Out of these 18 patients (100%), 10 (55.55%) and 8 (44.44%) were from group 2 and group 1, respectively. Of the 10 (100%) excluded patients in group 2, 3 (27.2%) died in the first 48 hours of intubation, 4 (40%) patients were not given consent to continue the study, and 3 (27.2%) patients were unlikely to need intubation for at least 48 hours. Out of 8 (100%) excluded patients in group 1, 4 (50%) patients died in the first 48 hours of intubation and 4 (50%) patients were unlikely to need intubation for at least 48 hours.
Statistical Package for the Social Sciences (IBM SPSS Statistics 20.0, Somers, NY, USA) software was used for statistical analysis. Kolmogorov–Smirnov test was used for normality test of data distribution. To compare differences between the groups, Student’s t-test (normal distribution) and Mann–Whitney U test (non-Gaussian distribution) were used for continuous variables, and chi-square (χ2) test was used for categorical variables. The mean age (P = 0.25), mean sex (P = 0.120), mean APACHE II score (P = 0.65), risk factors VAP (P = 0.56), reasons for admission to the ICU (P = 0.120), presence of oral prosthesis (P = 0.64), and oral hygiene status (P = 0.3) were all noted. There were no significant differences between the 2 groups in the first admission ( Table 2 ).
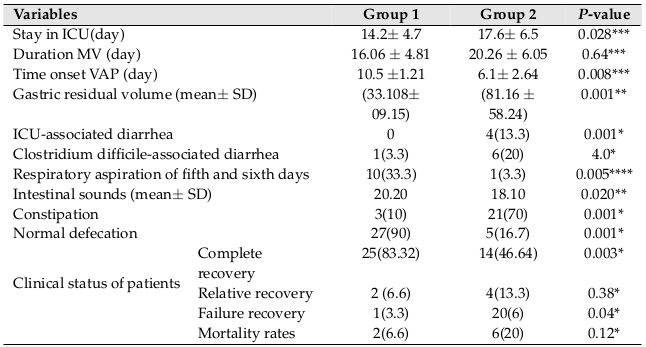
The primary outcomes of the present study was to evaluate the incidence of VAP, as analyzed 6 by generalized Wilcoxon test. Of the 60 patients who completed the study, 22 patients were diagnosed with VAP [group 1: 7 patients (23.3 %); vs. group 2: 15 patients (50 %); p=0.03]. In this study, the addition of fenugreek seeds in the daily diet led to a significant reduction in cases of VAP.
Secondary outcomes included the average of days spent in the ICU [group 1: 14.2±4.7 vs. group 2: 17.6±6.5; p=0.028], length of stay in hospital [group 1: 24.1±5.6 vs. group 2: 27.4±6.6; p=0.041], and rates of Clostridium difficile-associated diarrhea [group 1: 1(3.33%) vs. group 2: 6(20%); p = 0.04] were different between the two groups. The mean gastric residual volume during the first two days was not significantly different between the two groups but showed a strong trend to decrease in group 1 (one-way ANOVA showed a significant difference between both groups (p=0.001)). Analysis of cases of respiratory aspiration (33.3% vs. 3.3%; for group 2 vs. group 1; p=0.005) and mean time to occurrence of VAP (10.5±1.02 vs. 6.1±2.6, for group 2 vs. group 1; p=0.008) revealed that they are higher in the group 2. Duration of MV (16.06 ± 4.81 vs. 20.26 ± 6.05, for group 2 vs. group 1; p=0.64) was not different between the groups. No side effects attributable to the use of fenugreek seeds were observed. Specifically, no cases of diarrhea related to ICU were seen. There were 3 cases of constipation seen in group 1, while both cases were significantly increased in group 2, p=0.001 ( Table 2 ).
In relation to the clinical situation of patients, the results showed that complete recovery (83.3% vs. 46.6%; p=0.028] was significantly higher in group 1. Lack of recovery was significantly lower in group 1 (3.3% vs. 20%; p=0.04). Although the mortality rate was lower in group 1 than group 2 (2.2% vs. 20%; p=0.12), there was no significant difference between the two groups.
Discussion
The present study demonstrated that the use of fenugreek seeds has favorable effects on VAP, clostridium difficile-associated diarrhea, and other clinical outcomes (e.g. MV duration, length of stay in ICU of the critically ill patients, etc.). However, the changes in mortality are negligible. These findings were based on the findings of other scientists that fenugreek has a long history for treating gastrointestinal disorders and in cooking [23].
Karthiyaini et al. (2017) observed that the probiotic cells microencapsulated with alginate- fenugreek gum-locust matrix have enhanced viability than non-encapsulated cells during storage time of 3 months at 4◦C. Their tolerated digestive condition was efficient compared to non-encapsulated bacteria. These results demonstrate that fenugreek seeds act as a prebiotic [24]. Subsequently, the balance of gut flora enhances the innate immune system, which is associated with a reduction in the risk of VAP. Thus, the above results suggest and confirm that fenugreek seed powder diet may be promising for the management of critically ill VAP patients.
Glycemia control was associated with a reduction in risk of infection [25]; the results of the study, conducted by Kaur and et al (2016) [26], are pertinent to that study. In the study by Kaur et al., fenugreek seed powder was used as an add-on therapy along with metformin and evaluated in 60 Type 2 diabetes mellitus (DM) patients for 12-week duration. At the end of 12 weeks, the intervention group (in which 1 g of fenugreek seed powder was used as an add-on therapy to the metformin) showed a statistically significant decrease in fasting, postprandial blood sugar levels and HbA1c levels, as compared to those in the control group who received only metformin (p < 0.05) [26].
According to centers for disease control and prevention, prophylaxis of a gastrointestinal ulcer with non-alkaline medication is recommended to reduce VAP. The mucilage in fenugreek seeds assist in soothing gastrointestinal inflammation and coat the stomach and intestinal lining. A study conducted by Disilvestro et al. (2010) confirms the results of our study. In Disilvestro’s study, the authors compared the effects of fenugreek fiber and ranitidine tablets on heartburn symptoms. The results of their two-week study showed that in terms of heartburn symptoms, the fenugreek fiber and ranitidine groups showed significant differences from the placebo group; however, there was no significant difference between the fenugreek and ranitidine groups [27].
Wang et al. (2013) conducted a meta-analysis involving 844 patients (423 in the prebiotic group and 421 in the control group) in relation to the use of probiotics to prevent VAP. The results of their meta-analysis study indicated that VAP was reduced in the probiotic group, as compared with the control group. However, the difference was not significant, even over a total of 5 studies. Most of the results of this meta-analysis (e.g. ICU stay, hospital stay, duration of mechanical ventilation, and diarrhea) were consistent with our results [28].
Probiotics may be ineffective before persisting in the lower digestive tract. Probiotics cannot be general effects and their viability in gut environment is different. Prebiotics are a special form of dietary fiber that cannot be digested; specifically, they act as a fertilizer for good bacteria present in any individual’s gut [4]. On the other hand, administration of some probiotic strains in severe acute pancreatitis did not reduce the risk of infectious complications and was associated with an increased risk of mortality [8][9].
Our study is unique in that it demonstrates that use of fenugreek seeds can significantly reduce residual volume and respiratory aspiration, and eventually lead to a reduction in VAP. Our study also differs from previous studies in that we conducted a double-blinded study with coded gavage bowls and promoted the growth of good bacteria specific to each person’s gut by the use of fenugreek seeds.
This study had multiple limitations. First, to examine secondary endpoints, sample size was not large enough and the enlistment period was too long. Second, the data were gained from a single zone with innate prejudices related to local habits and population. Third, there was a need to enroll patients who were expected to have been under ventilation for at least 48 hours with a high risk for VAP. These issues indicate that the results of this study cannot be assigned to all ICU patients. In fact, the data interpretation is for patients with a high risk for VAP. Collectively, these data suggest that use of fenugreek seeds may be safe, nutritional, inexpensive, and a non- antibiotic procedure to prevent VAP in ICU patients. As a result, use of fenugreek seeds in the diet of patients undergoing long-term intubation is recommended.
Conclusion
In this study, we demonstrated the effect of fenugreek seed powder as an adjunct to other drugs in the prevention and treatment of VAP. According to our study fenugreek seed powder when used in the diet of ICU patients, the cases of VAP were cut in half. Treatment led to a statistically significant reduction in some complications of mechanical ventilation. Therefore, we conclude that fenugreek seed powder diet may be a promising addition for the management of critically ill patients.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
ESRD: End-Stage Renal Disease; HDNT: Hemodialysis Novel Therapies; NIDDK: National Institute of Diabetes and Digestive and Kidney diseases; RCT: Randomized, Controlled Trial; VAP: Ventilator-Associated Pneumonia.
Ethics approval and consent to participate
The Institutional Review Board of Sabzevar University approved the protocol of the present study (Code of ethics: IR.MEDSAB.REC.1394.128)
Availability of data and materials
Authors will provide if requested.
Competing interests
The authors declare that they have no competing interests.
Funding
Sabzevar University of Medical Sciences, Sabzevar, Iran.
Authors’ contributions
All authors contributed to the design of the research. AK, AZ, MR and YT collected the. Data. AZ and YT conducted analysis and interpretation of data. All authors drafted. The first version. AK, ZKH, AZ, MR and YT edited the first draft. All authors reviewed, commented and approved the final draft.
References
-
A
Torres,
MS
Niederman,
J
Chastre,
S
Ewig,
P
Fernandez-Vandellos,
H
Hanberger,
M
Kollef,
GL
Bassi,
CM
Luna,
I
Martin-Loeches.
International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator- associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). European Respiratory Journal.
2017;
50
:
1700582
.
View Article Google Scholar -
P
Bodera.
Influence of prebiotics on the human immune system (GALT). Recent patents on inflammation allergy drug discovery.
2008;
2
:
149-153
.
View Article Google Scholar -
NJ
Klingensmith,
CM
Coopersmith.
The gut as the motor of multiple organ dysfunction in critical illness. Critical care clinics.
2016;
32
:
203-212
.
View Article Google Scholar -
P
Shokryazdan,
MF
Jahromi,
B
Navidshad,
JB
Liang.
Effects of prebiotics on immune system and cytokine expression. Medical microbiology and immunology.
2017;
206
:
1-9
.
View Article Google Scholar -
W
Manzanares,
M
Lemieux,
PL
Langlois,
PE
Wischmeyer.
Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Critical Care.
2016;
20
:
262
.
View Article Google Scholar -
W
Kruis.
Probiotics. Digestive Diseases.
2013;
31
:
385-387
.
View Article Google Scholar -
S
Doron,
DR
Snydman.
Risk and safety of probiotics. Clinical Infectious Diseases.
2015;
60
:
S129-S134
.
View Article Google Scholar -
LE
Morrow,
MH
Kollef,
TB
Casale.
Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. American journal of respiratory and critical care medicine.
2010;
182
:
1058-1064
.
View Article Google Scholar -
H
Weng,
JG
Li,
Z
Mao,
Y
Feng,
CY
Wang,
XQ
Ren,
XT
Zeng.
Probiotics for Preventing Ventilator-Associated Pneumonia in Mechanically Ventilated Patients: A Meta-Analysis with Trial Sequential Analysis. Frontiers in pharmacology.
2017;
8
:
717
.
View Article Google Scholar -
M
Ouzir,
KE
Bairi,
S
Amzazi.
Toxicological properties of fenugreek (Trigonella foenum graecum). Food and Chemical Toxicology.
2016;
96
:
145-154
.
View Article Google Scholar -
JN
Losso,
DL
Holliday,
G
Richard,
N
Karki,
JW
Finley.
The health benefits of fenugreek-enriched cereal products. Cereal foods world.
2010;
55
:
236-241
.
-
FA
Toppo,
R
Akhand,
AK
Pathak.
Pharmacological actions and potential uses of Trigonella foenum-graecum: A review. Asian J Pharm Clin Res.
2009;
2
:
29-38
.
-
Gut Microbiome and p-Inulin in CKD - TarGut CKD Study (TarGut).
.
-
RR
Walli,
RA
Al-Musrati,
HM
Eshtewi,
FM
Sherif.
Screening of antimicrobial activity of fenugreek seeds. Pharmacy and Pharmacology International Journal.
2015;
2
.
-
RC
Garg.
Fenugreek: Multiple Health Benefits. 2016
.
-
CO
Olaiya,
KO
Soetan.
A review of the health benefits of fenugreek (Trigonella foenum- graecum L.): Nutritional, Biochemical and pharmaceutical perspectives. American Journal of Social Issues and Humanities.
2014
.
-
N
Neelakantan,
M
Narayanan,
RJ
de Souza,
RM
van Dam.
Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutrition journal.
2014;
13
:
7
.
-
X
Hu,
JS
Lee,
PT
Pianosi,
JH
Ryu.
Aspiration-related pulmonary syndromes. Chest.
2015;
147
:
815-823
.
View Article Google Scholar -
K
Shah,
J
Guarderas,
G
Krishnaswamy.
Aspiration-induced pulmonary syndromes. Annals of.
2016;
Allergy
:
Asthma Immunology 117
.
View Article Google Scholar -
O
Laila,
I
Murtaza,
MZ
Abdin,
S
Showkat.
GERMINATION OF FENUGREEK SEEDS IMPROVES HYPOGLYCAEMIC EFFECTS AND NORMALIZES INSULIN SIGNILLING PATHWAY EFFICIENTLY IN DIABETES. International Journal of Pharmaceutical Sciences and Research.
2016;
7
:
1535
.
-
MB
Didarshetaban,
S
Pour,
H
Reza.
Fenugreek (Trigonella foenum-graecum L.) as a valuable medicinal plant. International Journal of Advanced Biological and Biomedical Research.
2013;
1
:
922-931
.
-
A
Avalos-Soriano,
RD
la Cruz-Cordero,
JL
Rosado,
T
Garcia-Gasca.
4-Hydroxyisoleucine from Fenugreek (Trigonella foenum-graecum): Effects on Insulin Resistance Associated with Obesity. Molecules.
2016;
21
:
1596
.
View Article Google Scholar -
M
Blumenthal,
A
Goldberg,
J
Brinckmann.
Integrative medicine communications. Herbal medicines, Austin.
2000;
:
419-423
.
-
K
Damodharan,
SA
Palaniyandi,
SH
Yang,
JW
Suh.
Co-encapsulation of lactic acid bacteria and prebiotic with alginate-fenugreek gum-locust bean gum matrix: Viability of encapsulated bacteria under simulated gastrointestinal condition and during storage time. Biotechnology and Bioprocess Engineering.
2017;
22
:
265-271
.
View Article Google Scholar -
MH
Murad,
JA
Coburn,
F
Coto-Yglesias,
S
Dzyubak,
A
Hazem,
MA
Lane,
LJ
Prokop,
VM
Montori.
Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. The Journal of Clinical Endocrinology Metabolism.
2012;
97
:
49-58
.
View Article Google Scholar -
M
Kaur,
N
Singh,
G
Sharma,
D
Singh.
To study the efficacy and tolerability of fenugreek seed powder as add-on therapy with metformin in patients of type-2 diabetes mellitus. International Journal of Basic Clinical Pharmacology.
2016;
5
:
378-383
.
View Article Google Scholar -
RA
DiSilvestro,
MA
Verbruggen,
EJ
Offutt.
Anti-heartburn effects of a fenugreek fiber product. Phytotherapy Research.
2011;
25
:
88-91
.
View Article Google Scholar -
C
Yue,
W
Tian,
W
Wang,
Q
Huang,
R
Zhao,
Y
Zhao,
Q
Li,
J
Li.
The impact of perioperative glutamine-supplemented parenteral nutrition on outcomes of patients undergoing abdominal surgery: a meta-analysis of randomized clinical trials. The American Surgeon.
2013;
79
:
506-513
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 5 (2018)
Page No.: 2287-2295
Published on: 2018-05-19
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6612 times
- Download PDF downloaded - 2218 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress