Abstract
Introduction: Asian countries have the highest burden of liver diseases. Polygonum plebeium (P. plebeium), a.k.a. the common knotweed, is a species of plant in the knotwood family that can act as a blood purifier and has been widely used in Pakistan to cure liver disorders like jaundice and hepatitis. The plant is also used in the treatment of pneumonia, bowel complaints, diarrhea, dysentery, eczema and ring worms. Tannins, flavonoids, saponins and alkaloids are the major components of P. plebeium. Since its use in folk medicine in Pakistan, there has been little scientific evidence or information on it. Therefore, this study was aimed at investigating the anti-fibrotic effects of P. plebeium in carbon tetrachloride (CCl4)-induced hepatic toxicity and fibrosis.
Methods: The extracts of whole plant of P. plebeium were prepared and administered by oral gavage in rats. Liver fibrosis was induced by intraperitoneal (i.p.) administration of CCl4. To evaluate the hepatoprotective activity of P. plebeium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase (γGT) levels were quantified. Histological evaluation of liver tissue revealed tissue necrosis and extracellular matrix deposition. Real-time PCR was done to evaluate mRNA expression of genes related to liver fibrosis.
Results: The groups treated with P. plebeium extract showed an ablation in liver damage; the elevated enzyme levels of ALT, AST and γGT were decreased. Treatment with P. plebeium extract treatment restored the CCl4-induced tissue fibrosis by significantly suppressing alpha-smooth muscle actin (α-SMA), tumor growth factor beta (TGF-β) and collagen mRNA expression levels. Histology of liver sections also showed that the CCl4-induced fibrosis was improved in the treatment groups.
Conclusion: Polygonum plebeium has therapeutic potential and can be used for preventing fibrosis in inflammatory liver disease.
Background
Asian countries carry the highest burden of liver diseases worldwide [1]. Chronic liver disease related mortality in Pakistan mainly stems from chronic hepatitis C infection followed by metastatic adenocarcinoma, hepatocellular carcinoma, and cirrhosis [2]. Cirrhosis, the advanced stage of fibrosis, is inevitably linked to chronic liver diseases such as viral hepatitis. Many causative agents other than viruses also trigger inflammation which result in degeneration of hepatocytes and liver dysfunction [3]. Thus, cirrhosis is an irreversible form of fibrosis and the end stage of many diseases affecting the liver [4].
On a cellular level, liver fibrosis is caused by amassing of extracellular matrix proteins, such as collagen and fibronectins, produced by activated hepatic stellate cells [5]. These activated hepatic stellate cells are characterized by elevated mRNA expression of transforming growth factor beta (TGF-β), alpha smooth muscle actin (α-SMA), and collagen. Therefore, targeting phenotypically activated hepatic stellate cells is the mainstay of anti-fibrotic therapies.
A small number of drugs have been successfully used for the treatment of chronic liver diseases [6]. However, they are expensive and bear unavoidable side effects [7] . Attention is being paid to herbal remedies owing to their economy, ease of accessibility, and minor or no side effects. Herbal medicines are widely used by one quarter of hepatic patients [8].
Polygonum plebeium R.Br. (P. plebeium), also known as small knotweed, consists of tannins, essential oils, flavonoids, unsaturated sterols, triterpenoids, saponins and alkaloids [9]. It belongs to the family Polygonaceae [10]-[12]. It is one of the important plants having indigenous names such as “Chemti Sag”, “Dubia Sag” [13], “Anjaban” [9], and Lalbuti [12]. However, bistort [10] and small knotweed are the common English names of this plant. It is triangular in shape with approximate length and width of 1.4 mm and 1.0 mm, respectively. The shape is like that of a pyramid [14] ; the plant grows in the less waterlogged parts of swamps and thus it is commonly known to be a frequent wetland plant [15].
P. plebeium has exhibited anti-oxidant activity in in vitro studies [16]. The plant is commonly used for treating pneumonia, bowel complaints, diarrhea [10][13], dysentery [17], eczema, and ring worms [18]. It is found to cure dysentery when crushed with the bark of Butea superba and adventitious roots of Ficus benghalensis [17] . Balm made from the crushed leaves is used for treatment of eczema and ring worms [19]. The plant is used as a vegetable and tends to possess vitamin C [18]. Its roots are also used to treat bowel complaints. The ash and oil are used in eczema treatment; the powdered herb is used in pneumonia treatment [9], and the decoction of the plant root is used as a cooling agent [12]. P. plebeium is a blood purifier and has been widely used in Pakistan to cure liver disorders like jaundice and hepatitis [20]. However, no scientific evidence is available yet that demonstrates the mechanism of action of P. plebeium in the treatment of liver disorders.
Therefore the purpose of this study was to evaluate the antifibrotic and hepatoprotective mechanisms of P. plebeium extracts on carbon tetrachloride (CCl4)induced hepatic damage and fibrosis in rats.
Methods
Chemicals
Carbon tetrachloride (CCL4), isopropanol, chloroform, ethanol, hematoxylin, phosphomolybdic acid and acetic acid were purchased from Sigma. All the solvents used were of analytical grade.
Plant material
P. plebeium R.Br. (local name: Gorakh pan; plant official name reaffirmed from www.plantlist.org site on 18 Oct 2016) is widely distributed in Pakistan, India, Bangladesh, Sri Lanka and Madagascar [9] ( Figure 1 ). It was collected from a local herbal practitioner’s shop in Lahore and was identified from a herbarium at Punjab University; a voucher number was issued (Specimen #LAH672015). The whole P. plebeium plant was washed and air dried until it was moisture free, and then grinded in a mechanical grinder. Next, 850 g of the powdered plant was macerated with 5.5 L of ethanol: water (70:30) mixture, followed by filtration through vacuum suction pump. The solvent was separated using a rotary evaporator at 125 rpm and 37° until a sticky oily mass was separated from the mixture. The waxy material (PGOE) was filter separated and the filtrate (PGAE) was evaporated to semisolid consistency.
The organic layer (PGOE) was removed and dried, an emulsion was made using tragacanth and distilled water, and the emulsion was stored at 4oC. The aqueous layer and organic emulsion were further diluted with distilled water to form a working dilution of final concentration of 25 mg/mL.
Animals
The animal experimental protocol was approved by the Animal Research Ethics Committee of the Pharmacy Department of the University of Lahore (ref# IAEC2014003). Wistar rats (n=24) of both sexes weighing from 150-250 g were obtained from the Animal House of the University of Lahore and were kept under standard laboratory conditions (i.e. 25° and humidity of 45-55%). The animals were provided with food & water ad-libitum.
Induction of fibrosis
Liver fibrosis was induced by CCl4 according to an established protocol [21]. Briefly, CCl4 was injected intraperitoneally (i.p.) at a dose of 0.25 µL per gram of rat body weight twice a week during the 1st week, 0.50 µL/g twice a week for the 2nd week, and 1 µL/g body weight twice a week from weeks 3 to 6. Treatment with P. plebeium extract was started from week 3 with concomitant i.p. administration of CCl4. Wistar rats (of both sexes) were randomly divided into the following groups. Notably, Group I (n=4) was the control group (i.e. received
i.p. administration of corn oil as vehicle control), Group II (n=4) was the CCL4 group (i.e. administered i.p. with CCl4 as mentioned above), Group III (n=4) consisted of rats bearing CCl4−induced liver toxicity that were treated with aqueous extract of P. plebeium (250 mg/kg), Group IV (n=4) consisted of rats bearing CCl4−induced liver toxicity that were treated with organic extract of P. plebeium (250 mg/kg), Group V (n=4) consisted of the control group treated with corn oil and the aqueous extract of P. plebeium (250 mg/kg), and Group VI (n=4) was the control group treated with corn oil and the organic extract of P. plebeium (250mg/kg). Corn oil and CCl4 were given i.p. and the extracts were given orally using oral gavage. Since most studies used 100-500 mg extract per kg of body weight [22]–[24], the dose of 250 mg/kg was, therefore, selected for this study. The body weights (in grams) of the rats were recorded biweekly for six weeks of the experiment.
Biochemical evaluation
At the end of the experiment, the rats were euthanized by chloroform anesthesia. Blood samples were collected by cardiac puncture and centrifuged immediately for serum separation at 4000 rpm for 5 min, and for other blood analyses. Liver damage was assessed by estimation of serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma- glutamyl transpeptidase (γGT) using commercially available test kits and the kinetic IFCC method (NDI Europa GmbH, Germany). The results were expressed as international units/liter (IU/L).
Histopathological evaluation
Liver sections were collected and fixed with 4% formalin at room temperature. Four µm thick sections were cut for Masson’s Trichrome staining and Hematoxylin & Eosin (H&E) staining according to previous protocols [25][26]. The liver sections were evaluated histologically with a camera attached to a light microscope.
Total RNA Isolation, complementary DNA synthesis and Polymerase Chain Reaction
Large lobules of liver samples were collected and stored at -80°C for PCR analysis. Total RNA was extracted from a small piece of frozen rat liver using WizolTM reagent, followed by cDNA synthesis using M-MLV cDNA synthesis kit (Enzynomics, Inc., Korea). Real-time PCR was conducted using TOPrealTM qPCR 2X PreMIX (SYBR Green with low ROX) by Enzynomics using primer sets and the methods previously described [21]. Real-time PCR data was analyzed by measuring relative expression and standard deviation as previously described [27].
Statistical analysis
The results are shown as mean with standard deviation. GraphPad Prism (version 5) was used to analyze the data. One-way analysis of variance with post hoc Bonferroni correction test was applied to compare the mean of groups; p<0.05 was considered significant.
Results
Weight variation
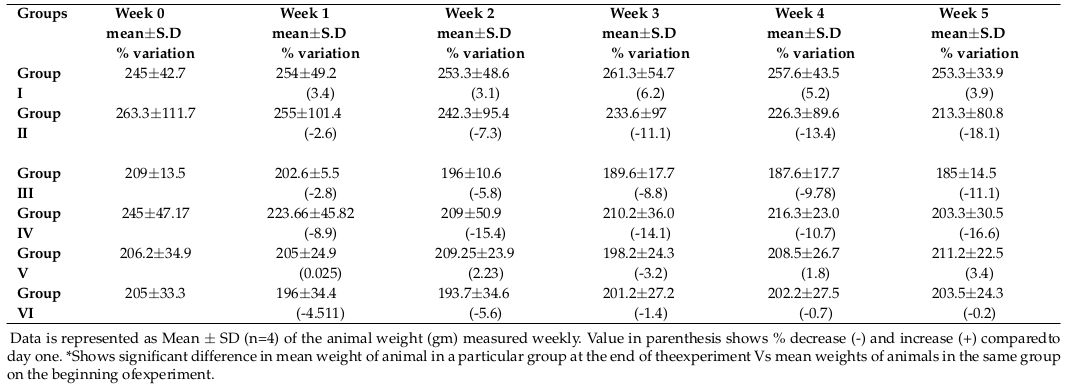
Table 1 shows the weight variation (in grams) of animals from each group measured bi-weekly before dosing of animals. There was a non-significant increase in weight up to 3.8% from day one in the control group. In contrast, there was a non-significant decrease in weight to -18.1% compared to day 1 for the CCL4 treated group. The weight variation for all other groups also did not show any significant change.
Evaluation of liver damage
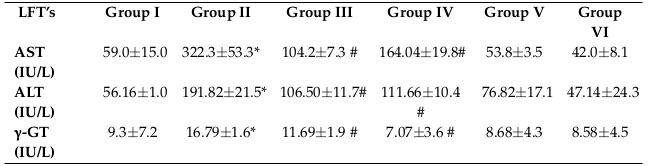
Table 2 shows the results of the liver assays. Elevated levels of serum aminotransferases (ALT and AST) and γGT are the major indicators of liver injury. Liver markers were significantly higher (P<0.05) in the CCl4 treated group than the control group. The average values (mean±stdev; in IU/L) for the control group were 59.0±15.0, 56.2±1.0 and 9.3±7.2 for AST, ALT and γGT, respectively. On the contrary, the mean values (in IU/L) for the CCl4 treated group increased to 322.3±53.3, 191.82±21.5 and 16.79±1.58 for AST, ALT and γGT, respectively. The average values (in IUL/L) of AST, ALT and γGT in Group III were 104.2±7.3, 106.50±11.7 and 11.69±1.9, respectively. In Group IV, the average values (in IU/L) for AST, ALT and γGT were 164.04±19.8, 111.66±10.4 and 7.07±3.57, respectively. The levels of AST, ALT and γGT in group V and VI were not much different from the control (Group 1).
Evaluation of blood cell indices
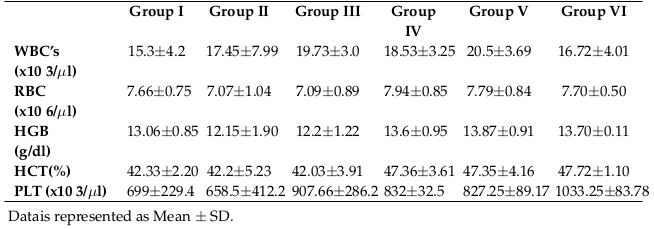
Table 3 shows the complete blood count (mean values) from each group. Count of white blood cells, red blood cells and platelets were not changed among the groups. Similarly, hemoglobin and hematocrit values remained the same among the groups.
Gene expression analysis of fibrosis-related genes
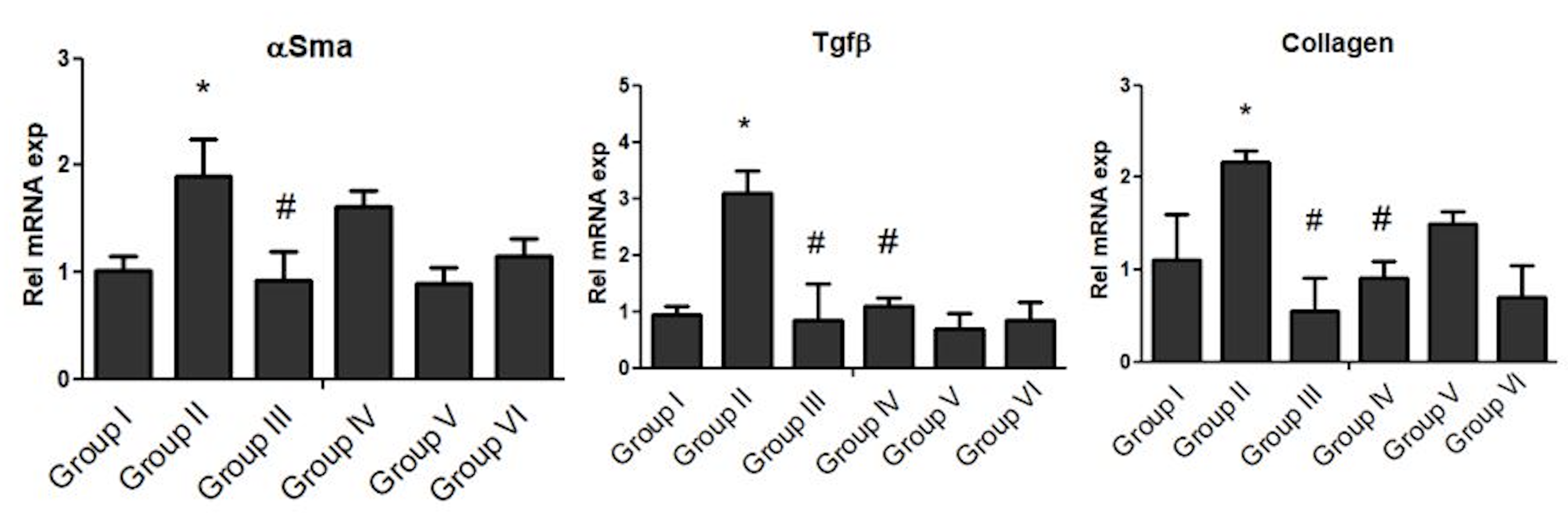
Gene expression of αSMA, TGF-β and collagen was assessed for all groups ( Figure 2 ). CCl4 treatment in Group II animals caused a significant increase in mRNA level of αSMA (p<0.05), TGF-β (p<0.0001) and collagen (p<0.0001), compared to Group I. Treatment with P. plebeium aqueous extract (PPAE; Group III), after three weeks of CCl4, significantly prevented mRNA expression levels of αSMA (p<0.05), TGF-β (p<0.001) and collagen (p<0.001). Likewise, in animals treated with P. plebeium organic extract (PPOE; Group IV), the mRNA levels of TGF-β (p<0.0001) and collagen (p<0.0001) remained lower compared with the levels in Group II (CCl4 treated group). However, no repression was observed in αSMA expression following treatment with PPOE Figure 2 .
Histopathological Evaluation
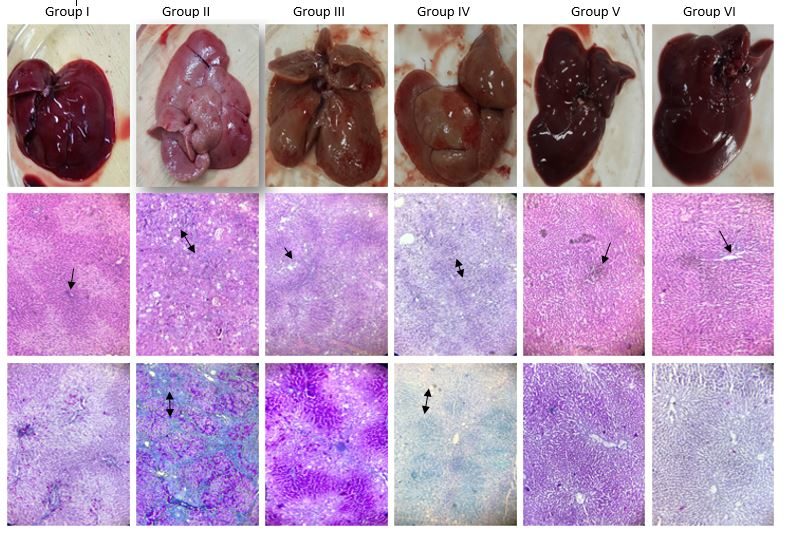
Gross and microscopic histopathological features of the liver in the various treatment groups are shown in Figure 3 . The top panel shows the liver images after excision from the animals.
Control (Group I) showed healthy liver with soft, smooth and shiny surface texture. However, liver of animals in the CCl4-treated group (Group II) showed a nodular appearance with rough, hard patchy surface and no shine. The faint red color indicated decreased blood flow through the liver. PPAE (Group III) and PPOE (Group IV) treated rats were comparatively healthy; the livers from these groups were similar in color but with a smoother surface than those in the CCl4- treated group (Group II). The livers of rats in the group treated with corn oil (control instead of CCL4) and P. plebeium aqueous extract (Group V) and Group IV rats were comparatively healthier, with bright, shiny and smooth surfaces. The middle panel shows H&E staining of the livers, where blue staining represents nuclei, pink represents cytoplasm, and deep pink or red represents muscle fibers. The central portal vein is shown by a one-sided black arrow and cellular necrosis is indicated by double-headed arrows. The liver section of the control group and the extract only group showed normal central vein and radiating hepatocytes with no histopathological changes. Liver sections from the CCl4-treated rat show vacuolization of hepatocytes, inflammatory cell infiltration and hepatocyte necrosis. Liver sections treated with aqueous extract + CCl4 showed inflammatory cell infiltration with normal hepatocytes. Liver sections treated with organic extract + CCl4 showed inflammatory cell infiltration and fat deposition with dispersed hepatocytes.
The lower panel shows the Masson’s trichrome staining of the liver sections. Blue stain indicates fibrous tissue. The degree of fibrosis tissue (collagen) accumulation is directly related to intensity of the blue stain. Red indicates hepatocytes and dark red to black represents nuclei. The central portal vein is shown by a one-sided black arrow and collagen accumulation or fibrosis is indicated by double-headed arrows. The liver sections of control and extract only-treated rats showed normal liver morphology (i.e. central vein and radiating hepatocytes with no collagen accumulation). Liver sections from the CCl4-treated rats showed extensive fibrosis and fibrous bands, indicating very high collagen accumulation leading to nodule formation. On the contrary, the liver tissue of animals treated with aqueous extract showed hepatic plates which were more than 1-cell thick, indicating regeneration of hepatocytes. The liver sections of animals treated with organic extract showed portal inflammation with no bile duct injury.
Discussion
Liver is one of the vital body organs which detoxify toxic drugs and chemicals and is, therefore, prone to chemicals or microbe-induced inflammation. Liver fibrosis and cirrhosis due to viral hepatitis are main reasons for high mortality rate and disease burden worldwide, especially in developing countries [28]. CCl4 is widely used experimentally to study liver inflammation, fibrosis and cirrhosis [21][29]. It induces hepatic damage by the formation of free radicals during reductive dehalogenation by cytochrome P-450 during its metabolism which causes lipid peroxidation of cellular membranes, leading to necrosis. The initial events of CCl4 evoke the secondary mechanisms which ultimately disrupt the plasma membrane and cause cell death [30][31].
The efficacy of any hepato-protective drug is mainly dependent on how effectively it reverses the toxic effects or restores normal hepatic physiological function. The study herein showed that P. plebeium extract treated groups had considerable protection against toxicants, as evidenced from the decrease of elevated enzyme levels of ALT, AST and γGT in the treated groups Table 2 . The evident decrease in mean values (mean±stdev; in IU/L) of serum aminotransferases (ALT, AST) and γGT indicates the effectiveness of the extract in restoration of normal liver function. Our results are in accordance with earlier studies which showed that plant extracts possess antioxidant properties and have hepatoprotective effects in CCl4-induced model of liver injury [21][29].
CCl4 toxicity induces oxidative stress which leads not only to hepatocyte damage but also to release of cytokines such as TGF-β. TGF-β stimulates rat hepatic stellate cells (HSC) and helps them differentiate into myofibroblast-like cells, which deposit extracellular matrix and collagen [29]. The HSCs activated by the reactive oxygen species and various cytokines in an accelerated fashion lead to liver fibrosis. Treatment with P. plebeium extracts attenuated the CCl4-induced functional impairment and liver fibrosis, including reversal of HSC activation as demonstrated by suppressed expression of α-SMA, TGF-β and collagen mRNA ( Figure 2 ). These results are also in line with earlier studies [21][29].
Histological staining for extracellular matrix deposition and hepatocyte morphology ( Figure 3 ) also show the potential use of P. plebeium for the treatment of liver fibrosis. The histological assay indicated that treatment with extracts helped in recovering the structural integrity of liver cells. Liver injury activates HSCs to proliferate and produce extracellular matrix which ultimately leads to fibrosis. The amount of collagen deposition was demonstrated by Masson’s trichrome staining of the tissue sections ( Figure 3 ). The liver sections of rats treated with CCl4 showed fatty changes and hepatocyte necrosis. Appearance of fatty droplets in the liver parenchyma as a result of CCl4 intoxication occurred at an earlier stage than extracellular matrix deposition. From the histopathological studies it is evident that the extracts slowed the fibrosis process, including apoptosis of HSC, by minimizing the disruption of hepatocyte structure and accelerating hepatic regeneration. Overall, the histology of liver sections indicated that the fibrosis resolved to a level close to control after treatment (Group III and IV).
P. plebeium contains a variety of phytochemicals including flavonoids and alkaloids [9]. Flavonoids possess significant free radical scavenging activities while alkaloids possess diverse pharmacological activities, including anti-inflammatory and anti-proliferative functions. Also, plants which contain polyphenols and flavonoids have been shown in vivo to have hepatoprotective and anti-inflammatory actions [29]. Clinical studies have also shown that liver cholestasis can be treated with antioxidants [32][33]. All these studies suggest that P. Plebeium exerted its anti-fibrotic effects by virtue of its alkaloid and flavonoid contents by interfering and preventing CCl4-induced hepatic inflammation and fibrosis.
In conclusion, this study shows that P. plebeium may be used as a natural drug for the treatment of liver fibrosis. Indeed, this study encompasses some limitations which include the use of single dose of extract, lack of standard hepatoprotective drug (or some antioxidant as a control), and lack of chemical characterization of the extracts. However, regardless of the above mentioned limitations, the data from the study herein are significant; they indicate the protective effects of
P. plebeium extracts in CCl4-induced toxicity in rat liver. As well, the data show repression of deposition of extracellular matrix after treatment with P. plebeium extracts.
Conclusion
The current study demonstrates the compelling hepatoprotective activity of P. plebeium extracts in opposing CCl4-induced toxicity on liver in a rat model. The protective effects may be from phytochemicals, such as flavonoids and alkaloids, which exert and maintain anti-inflammatory and antioxidant effects. Therefore, data from this study justifies the traditional use of P. plebeium for preventing liver disorders and associated fibrosis in patients.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, PPAE: Polygonum Plebium aqueous extract, PPOE: Polygonum Plebium organic extract, TGF-β: Transforming growth factor beta, αSMA: alpha smooth muscle actin, γGT: Gammaglutamyl transpeptidase
Ethics approval and consent to participate
Animal experiment protocol was approved by animal research ethics committee of the faculty of Pharmacy, The University of Lahore, ref # IAEC2014003.
Competing interests
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The research was conducted as a part of M-Phil research thesis of Ms Ayisha Waheed and content had been deposited in the HEC repository as a requirement of M-Phil Degree.
Authors’ contributions
Atta ur Rehman supervised the project, designed, analyzed and finally wrote the manuscript. Ayisha Waheed performed animal experiments, obtained raw data and wrote initial draft. Rabia Tariq and muhammad javaid Tahir assisted in gene expression analysis. MUhammad zaman helped in design of study, data analysis assisted in manuscript writeup.
References
-
AV
Boopathy,
KD
Pendergrass,
PL
Che,
YS
Yoon,
ME
Davis.
Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res Ther.
2013;
4
:
43
.
View Article PubMed PMC Google Scholar -
N
Khokhar.
Spectrum of chronic liver disease in a tertiary care hospital. JPMA. The Journal of the Pakistan Medical Association.
2002;
52
:
56-58
.
PubMed Google Scholar -
K
Sembulingam,
P
Sembulingam.
Essentials of medical physiology. 2012
.
-
PA
McCormick.
Hepatic Cirrhosis. 2011
.
-
R
Bataller,
DA
Brenner.
Liver fibrosis. The Journal of clinical investigation.
2005;
115
:
209-218
.
View Article PubMed PMC Google Scholar -
P
Muriel,
Y
Rivera-Espinoza.
Beneficial drugs for liver diseases. Journal of Applied Toxicology.
2008;
28
:
93-103
.
View Article PubMed Google Scholar -
MW
Fried.
Side effects of therapy of hepatitis C and their management. Hepatology.
2002;
36
.
-
F
Stickel,
D
Schuppan.
Herbal medicine in the treatment of liver diseases. Digestive and liver disease.
2007;
39
:
293-304
.
View Article PubMed Google Scholar -
AN
Hasan,
P
Roy,
NJ
Bristy,
SK
Paul,
TB
Wahed,
MN
Alam.
Evaluation of in vitro antioxidant and brine shrimp lethality bioassay of different extracts of Polygonum plebeium R. Br.. International Journal.
2015;
3
:
97-107
.
-
A
Jabeen,
MA
Khan,
M
Ahmad,
M
Zafar,
F
Ahmad.
Indigenous uses of economically important flora of Margallah hills national park, Islamabad, Pakistan. African Journal of Biotechnology.
2009;
8
.
-
JB
Kirkpatrick,
CE
Harwood.
Conservation of Tasmanian macrophytic wetland vegetation. 1983
.
-
S
Kumar,
F
Parveen,
S
Goyal,
A
Chauhan.
Indigenous herbal coolants for combating heat stress in the hot Indian Arid Zone. Indian Journal of Traditional Knowledge.
2008;
7
:
679-682
.
-
S
Banerjee,
D
Kar,
A
Banerjee,
D
Palit.
Utilization of some aquatic macrophytes in Borobandh-a lentic water body in Durgapur, West Bengal, India: Implications for socio- economic upliftment of local stakeholder. Indian J Appl Pure Biol.
2012;
27
:
83-92
.
-
C
KANTACHOT,
P
CHANTARANOTHAI.
Achene morphology of Polygonum sl (Polygonaceae) in Thailand. Tropical natural history.
2011;
11
:
21-28
.
-
MS
Chauhan,
MF
Quamar.
Mid-Holocene vegetation vis-à-vis climate change in southwestern Madhya Pradesh, India. Current Science(Bangalore).
2012;
103
:
1455-1461
.
-
YL
Ho,
SS
Huang,
JS
Deng,
YH
Lin,
YS
Chang,
GJ
Huang.
In vitro antioxidant properties and total phenolic. 2012
.
-
A
Dey,
JN
De.
A survey of ethnomedicinal plants used by the tribals of Ajoydha hill region, Purulia district, India. American-Eurasian Journal of Sustainable Agriculture.
2010;
:
280-291
.
-
N
Sreeramulu,
GD
Ndossi,
K
Mtotomwema.
Effect of cooking on the nutritive value of common food plants of Tanzania: part 1 - vitamin C in some of the wild green leafy vegetables. Food Chemistry.
1983;
10
:
205-210
.
View Article Google Scholar -
MB
Siddiqui,
MM
Alam,
W
Husain.
Traditional treatment of skin diseases in Uttar Pradesh, India. Economic Botany.
1989;
43
:
480-486
.
View Article Google Scholar -
S
Ahmad,
K
Alam,
HM
Wariss,
S
Anjum,
M
Mukhtar.
Ethnobotanical studies of plant resources of Cholistan desert, Pakistan. Int J Sci Res.
2014;
3
:
1782-8
.
-
A
Rehman,
M
Liaqat,
R
Asghar,
N
i Husain Syed.
Evaluation of methanolic extract of Phragmites karka on carbon tetrachloride-induced liver fibrosis in rat. 2017;
2017
:
12
.
-
AIAA
Alrheam.
Biochemical effects of Calotropis procera on hepatotoxicity. Biomedical Research and Therapy.
2015;
2
:
446-453
.
View Article Google Scholar -
M
Fiaz,
N
Fiaz,
L
Shakir,
A
Alamgeer,
W
Mehmood,
G
Mustafa,
A
Rauf,
K
Najam.
Hepatoprotective effect of a polyherbal formulation and ascorbic acid in paracetamol induced hepatic damage in rabbits. Biomedical Research and Therapy.
2017;
4
:
1261-1277
.
View Article Google Scholar -
M
Saleem,
A
Asif,
MF
Akhtar,
A
Saleem.
Hepatoprotective potential and chemical characterization of Artocarpus lakoocha fruit extract. 2018;
2018
:
13
.
-
JD
Bancroft,
A
Stevens,
DR
Turner.
Theory and practice of histological techniques: Churchill Livingstone New York. the text.
1996;
:
766
.
-
KS
Suvarna,
SK
Suvarna,
C
Layton.
Bancroft's Theory and practice of histological techniques, expert consult: Online and Print, 7: Elsevier Health Sciences. 2012
.
-
KJ
Livak,
TD
Schmittgen.
Analysis of relative gene expression data using real-time quantitative PCR and the 2? ??CT method. methods.
2001;
25
:
402-408
.
-
SMA
Shah,
SA
Mashia,
MF
Younus,
A
Ghauri,
R
Ejaz,
H
Alshalabi,
IK
Kakar,
M
Umar.
Hepatic cirrhosis-disease burden. J. Rawalpindi Med. Coll. Stud. Suppl.
2015;
19
:
17-20
.
-
DH
Jeong,
GP
Lee,
WI
Jeong,
SH
Do,
HJ
Yang,
DW
Yuan,
HY
Park,
KJ
Kim,
KS
Jeong.
Alterations of mast cells and TGF-?1 on the silymarin treatment for CCl(4)-induced hepatic fibrosis. World Journal of Gastroenterology : WJG.
2005;
11
:
1141-1148
.
View Article PubMed PMC Google Scholar -
RO
Recknagel,
EA
Glende,
JA
Dolak,
RL
Waller.
Mechanisms of carbon tetrachloride toxicity. Pharmacology & therapeutics.
1989;
43
:
139-154
.
View Article Google Scholar -
A
Eidi,
P
Mortazavi,
M
Bazargan,
J
Zaringhalam.
Hepatoprotective activity of cinnamon ethanolic extract against CCL 4-induced liver injury in rats. EXCLI J.
2012;
11
:
495-507
.
PubMed PMC Google Scholar -
M
Khoshbaten,
A
Aliasgarzadeh,
K
Masnadi,
MK
Tarzamani,
S
Farhang,
H
Babaei,
J
Kiani,
M
Zaare,
F
Najafipoor.
N-Acetylcysteine Improves Liver Function in Patients with Non- Alcoholic Fatty Liver Disease. Hepatitis Monthly.
2010;
10
:
12-16
.
PubMed PMC Google Scholar -
K
Mumtaz,
Z
Azam,
S
Hamid,
S
Abid,
S
Memon,
HA
Shah,
W
Jafri.
Role of N- acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatology International.
2009;
3
:
563-570
.
View Article Google Scholar
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 4 (2018)
Page No.: 2223-2234
Published on: 2018-04-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 10307 times
- Download PDF downloaded - 2422 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress