Abstract
Cervical cancer is one of the leading cancers in women worldwide especially in developing countries. Various etiological factors are described, of which Human papiloma virus (HPV) is proved by various molecular epidemiological studies to play a major role. However many co-factors are required and thought to facilitate the action of HPV in cervical carcinogenesis. Here the role of various viruses in cervical cancer and its implication in screening and diagnosis of cervical cancer is highlighted. In-depth knowledge of role of different viruses helps in better screening methods and probably in target therapy / development of an appropriate vaccine.
Introduction
Cervical cancer is the third common cancer in the world in females and fourth leading cancer for death in women. Every year 530,000 cases are diagnosed and 265, 653 deaths occur in the world due to cervical cancer. The incidence/prevalence is decreasing in developed countries due to health awareness and regular/ systemic cervical cancer screening; some countries using HPV DNA (Human Papilloma Virus) test in addition to Pap tests. This routine screening has reduced the incidence to 70% in developed countries. However, cervical cancer persists in developing countries that constitute 85% of total world cases, despite only 5% global cancer resources in these countries. The incidence in developing countries is 40 per 100,000 populations Jean Anderson, 2012Marinho- Dias and Sousa, 2013Ononogbu et al., 2013Sinayobye et al., 2014Wright et al., 2007.
In India, it ranks as second common cancer in females after oral cancer especially in rural and semi-urban population constituting 17% of total female cancers.
However in metropolitan cities breast cancer is taking the lead Kalyani et al., 2010. Every year 122,844 cases are diagnosed, and 64,477 deaths occur in India due to cervical cancer Kalyani et al., 2010.
Since 1990 many infectious agents are established as the etiological agents in various cancers in humans constituting approximately 15% of all cancers Marinho-Dias and Sousa, 2013. Human Papilloma Virus (HPV) is the proved major etiological factor in etiopathogenesis of cervical cancer. HPV is necessary but alone is not sufficient to cause cervical cancer as there is a long latent period between the infection, and manifestation of cervical cancer and all women infected with HPV does not have cervical cancer. Hence, there are some co-factors that have synergetic action with HPV in causing cervical cancer. The co-factors proved by epidemiological studies are smoking, nicotine, long-term use of oral contraceptives, use of intrauterine contraceptive device, lifestyle as early initiation of sexual life/multiple sexual partners, young age at full term pregnancy, multiple full-term pregnancy, low socioeconomic status, diet low in antioxidants, genetic predisposition and decreased immunity. In addition, the sexual transmitted diseases like Chlamydia trachomatis, Gardenella vaginitis, etc. has role in cervical cancer Alibek et al., 2012Marinho-Dias and Sousa,2013NobelPrize, 2008. In this article, the role of different viruses in the pathogenesis of cervical cancer is highlighted.
It took nearly 140 years to prove that the virus is the etiological factor in the pathogenesis of cervical cancer. In 1960 and 1970 Herpes Simplex Virus 2 (HSV-2) was thought to be the virus responsible for cervical cancer. HPV was discovered in 1980, which was later established as the primary etiological factor in cervical cancer in 1990. The other viruses described in literature are Human Immunodeficiency Virus (HIV), Cytomegalovirus (CMV), Human Herpes Virus 6 (HHV 6), Epstein Barr Virus (EBV) and Hepatitis C Virus (HCV) Alibek et al., 2012Krishnakumar Duraisamy, 2011Marinho-Dias and Sousa, 2013NobelPrize, 2008. In general the various mechanisms of actions of these viruses on cervix are: direct action by causing genetic alteration in host cells and giving rise to abnormally increased proliferation of cells; indirect action by decreasing apoptosis of altered cells and synergetic action with various physical and chemical carcinogens acting at cervix. These factors mainly act at squamocolumnar junction or transformation zone of the cervix where the immature cells have genetic imbalance are susceptible to malignant transformation Jones, 1995Krishnakumar Duraisamy, 2011.
Human papilloma virus (hpv)
It was Harald Zur Hausen, a German scientist; in 1974 put forth the hypothesis that chronic infection of the cervix by HPV is the cause of cervical cancer. Nearly after ten years later in 1983 he isolated HPV 16 in cervical cancer cells. Subsequently a year later Harald isolated HPV 18. The role of HPV in cervical cancer was established by various large-scale epidemiological studies and molecular techniques in 1990. In 2008, Harald received Noble prize for this discovery Jean Anderson, 2012NobelPrize,2008.
HPV is a non-enveloped, doubled stranded DNA virus. More than 100 subtypes are described, of which 40 subtypes infect cervix. There are 15 high-risk (HR HPV) types which are involved in cervical cancer are 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82. In persistent infection, the risk of precancerous lesion is 10-15% in HPV 16/18 infection and 3% in other highrisk types. HPV causes approximately 90% of all cancers in females. HR HPV causes 90-100% of cervical cancer, HPV 16/18 causes 70% of cervical cancer and HPV 16 alone causes 50% of cervical cancer. HPV 18 and 45 is more associated with adenocarcinoma of the cervix. HR-HPV is detected in 88% of squamous cell carcinoma, 95% of high-grade squamous intraepithelial lesion, 75% of low-grade squamous intraepithelial lesion, and 14% in normal smear. Multiple HPV infection in invasive cervical cancer ranged between 0- 36% and HPV 33 is common element in multiple infections because HPV 33 usually occurs in combination with phylogenetically closely related types as HPV 16, 31, 35 and 58. The virus has early genes (E1 to E7) that encode proteins that regulate transcription, replication and maintenance and take an important role in the early phase of cervical carcinogenesis. The late capsid genes (L1 and L2) encode structural proteins that are required to form viral proteins and hence in replication of the virus Abreu et al., 2012Grce et al., 2004Jones, 1995Kahla et al., 2012Khenchouche et al., 2013NobelPrize, 2008Villa and Denny, 2006.
HPV infection is a sexually transmitted disease. Vaginal intercourse is usually associated with microtrauma of cervical epithelium by which HPV gains entry into cervical epithelium, reaches and infects basal/ parabasal cells where it persists as episomal, integrated or mixed forms. Episomal forms are seen in 81.8% of benign cervical lesions and 37.5% in cervical cancer. Integrated forms are seen in 18.15 in benign lesions and 62.5% in cervical malignancy. HPV 16 is present exclusively in episomal forms in 30-70% of cervical cancer and HPV18 is reported to be mainly in an integrated form, thus indicating different biological characteristics of HPV. HPV 18 co-infection was more likely linked to HPV 16 integrated forms. HPV 18 infected patients show rapid progression through precancerous stages. Cancer with HPV 16 and 18 are more aggressive. HPV 33 was found more frequently associated with HPV 16 episomal forms than HPV 18. HPV types other than 16 and 18 are an independent predictor of better survival in patients with cervical carcinoma. The virus subsequently replicates and also causes cytological changes in the epithelial cells. The virus remains in the epithelial cells as it matures into intermediate and superficial keratinocytes and later shed off at which point the women is infectious. The natural history of HPV infection depends on persistence or clearance of infection. 90% of infection usually clears in the early phase of infection in two to three years usually in adolescence and young adults. If the infection persists, HPV gives rise to cervical intraepithelial lesion (CIN) which at any phase may regress or progress to cervical cancer ( Figure 1 ). The latent period reported for HPV detection to CIN3 is seven to eight years, from CIN1 to CIN2 is two to three years and from CIN3 to invasive carcinoma is five to seven years. About 40 % of young women have latent infection of which only 1% is having HR-HPV in cervical tissue develop cervical cancer. 12% of individuals with persistent HPV infection develop pre-cancer and cancer of the cervix. About 99% of invasive cervical cancer has been found to be HPV-positive. The peak age of invasive cervical cancer is 40-50 years of age Grce et al., 2004 http://www.virology.uct.ac.za/teachhpv.htmlJean Anderson,2012Kahla et al., 2012Ononogbu et al., 2013.
The molecular mechanism of HPV infection which transforms the epithelial cells into dysplasia or cancer cells begins with the integration of the viral genome to host genome with decreased expression of L1/2 protein and increased expression of E1/2 & E6/7 protein. E2 has a crucial role in a viral life as a transcription and a replication factor. E2 disruption is seen in 62.5% cases of SCC and 18.1% cases of benign lesions. The affinity of HPV viral proteins for the products of tumour suppressor genes differs depending on the oncogenic potential of HPV. E6 and E7 of HR-HPV bind pRb and p53 respectively with high affinity. The E6 protein caused the inactivation of P53 protein and increased expression of P14 protein (tumour suppressor genes). E7 protein caused the inactivation of Rb protein and increased expression of P16 protein (tumour suppressor genes). E7 protein of HR-HPV has 10 fold higher efficiency to bind Rb protein than E7 of low-risk HPV. Hence, E6 and E7 together causes early immortalization of epithelial cells, deregulation of cell cycle, chromosomal instability and cervical carcinogenesis. Inactivation of P53 protein causes decreased apoptosis, and that of Rb protein causes increased proliferation of cells. In addition, chronic cervicitis by HPV causes production reactive oxygen species and free radicals that cause oxidative stress followed by the negative impact on genetic and cellular processes Jean Anderson, 2012Jones, 1995Kahla et al., 2012Prayitno, 2006.
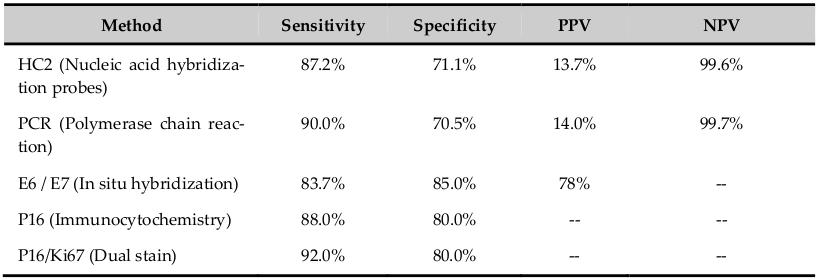
The HPV cannot be cultured. The infection can be detected by various methods as electron microscopy, immunology, serology and molecular technology; each method has different sensitivity and specificity ( Table 1 ). The nucleic acid probe methods are Southern & Northern blot, dot blot, in-situ hybridization, signal amplification method as Hybrid capture-2 and DNA sequencing. Viral capsid proteins (L1 &L2) are expressed in only productive infection. The detection of HPV E6 and E7 are more specific. HPV DNA suggests only the presence of the viral genome whereas Viral RNA assays suggest viral genome expression and hence the viral activity in the infected cells. The advantage of in-situ hybridization is, it can be used in formalin fixed paraffin embedded tissue sections. Hybrid Capture (HC), PCR and RT-PCR methods have high sensitivity and specificity which identifies different types of HPV. HC2 is USA FDA approved method and detects 13 HPV-HR and 5 low-risk types using RNA probes Villa and Denny, 2006. PCR detects more than 48 types by hybridization using typespecific probes. HPV DNA test is more sensitive (85%) and has excellent negative predictive value (97%) which is more important in women who undergo screening 1-2 times in her lifetime; however it has poor specificity (84%) and positive predictive value compared with Pap test. In HPV-positive women, the risk of developing cervical neoplasia is between 3-10 years. Hence, there is a suggestion to do HPV test first and if positive followed by cytology test. The risk of CIN II/III in HPV DNA positive and negative cytology report is 3-7%. Following HPV DNA negative test, sixyear risk of CIN III was as low as 0.27%. HPV genotyping is better than HPV DNA test. In 2009, US FDA approved genotyping test for HPV 16 and 18 (Cervista HPV 16/18). The risk of CIN III is 10% over 1-4 years and 2-5 years in women positive for HPV 16 and HPV 18 respectively Jin et al., 2013Villa and Denny, 2006.
The merits of HPV tests are; primary screening where it detects the HPV infection caused by high-risk or low-risk subtype alone or in combination with Pap test, test is objective, reduces the frequency of cervical cancer screening in routine as well as in repeat screening especially when combined with cytology test, more useful in women of 30 years or more where HPV infection persists, negative results gives greater reassurance to women, discriminates lesions caused by HR HPV which has high risk to progress to cancer, follow-up of women with abnormal screening test but negative with colposcopy/biopsy, indicates common subtypes existing in the given population and thus indicates the effectiveness of HPV vaccine which is a primary prevention and vaccination against HPV 16/18 potentially prevents more than two-third of cervical cancer worldwide, increases the quality of cervical cancer screening as secondary prevention and used to monitor post-treatment status to assess recurrent/residual lesion (for 8 years). In posttreatment cases, the sensitivity of cytology and HPV tests are 48.8% and 92.7% respectively. HPV test is also used for triage in ALTS and cases of atypical glandular cells cytology results. Thus, HPV test helps in diagnostic and prognostic use in clinical management in women with HPV-related cervical disease. The HPV test provides insight into the biology of HPV-induced cervical cancer and hopefully leads to the development of nonsurgical therapies. The demerits are; the test is not useful in adolescent and young adults to infection in this population is transient, positive test leads to unnecessary anxiety in the patients, due to low specificity there can be under/over treatment of patients, indicates only presence of virus in host cells (episomal/integrated) which may/may not progress to immortalization and abnormal proliferation of cells. The prevalence of HPV 16 and 18 reported is 10.7% in healthy cervix, 31.5% in cervicitis, 46.7% in CIN and 83.3% in squamous cell carcinoma. Hence the emergence of novel markers of HPV infection and cervical cancer screening that increase positive predictive value of current screening methods Abreu et al., 2012Apgar et al., 2009Jin et al., 2013Villa and Denny, 2006Wright et al., 2007Zhao et al., 2012.
The novel markers in HPV infection and cervical cancer screening are: L1/L2 proteins that are positive in early phase of infection and expression decreases with integration of viral genome to host genome; HRHPV E2 transcripts serve as an additional biomarker of cervical diseases and is more sensitive indicator of the genomic disruption than analysis of genomic DNA; E6/E7 proteins are expressed once there is integration of viral genome to host genome that has increased chance of progression and has increased sensitivity and specificity for cervical cancer screening; P53, Rb protein, P16, P14, P21, P27, Cyclins D/A/E markers are expressed following integration of viral genome to host genome with deregulation of cell cycle and immortalisation of epithelial cells which result in increase cell proliferation and decrease in apoptosis. The dual stains (cocktail markers) like P16/Ki67, P16/HPV L1 and BD-ProEXC are used which gives more information about molecular changes in HPV infected epithelial cells. P16/Ki67 indicates immortalization of HPV-infected cells and abnormal proliferation of these immortalised cells. P16/HPV L1 indicates HPV infection and progression to immortalization of these cells. BD-ProEXC is expressed when viral DNA integrates host genome indicating increased levels of E6 and E7 with aberration of S-phase induction. Table 2 shows the sensitivity, specificity, positive predictive value and negative predictive value of some novel markers. The novel markers in HPV infection play a greater role in cervical cancer screening Jin et al., 2013Kahla et al., 2012Villa and Denny, 2006.
Human Immunodeficiency Virus (HIV)
It is an RNA virus having lipid envelope. About 33.3 million individuals worldwide are living with HIV of which 50% are females. HIV and HPV have common route of transmission, i.e., by sexual contact, and both has synergetic role. There is increased risk of CIN/cervical cancer in HIV-infected women as evidenced in context with HPV infection by increased prevalence (24-76%) and incidence of infection, greater prevalence (3-5 times) of oncogenic subtype, persistent infection, less clearance of infection, reactivation of latent infection, acquisition of new HPV subtype infection, multiple HPV subtype infection, increased viral load and infection by less virulent subtypes causing risk of cancer. The rate of oncogenic HPV infection is high especially in cases with increasing HIV viral load and decreasing CD count of less than 200 indicating impaired cell-mediated immunity which is required for development of pre-cancerous lesion and progression of the disease. Each 100 cell/mm3 increase in CD4 cell count reduced the risk of low-grade lesions by 13%, and high-grade lesions by 18% Jean Anderson, 2012Ononogbu et al., 2013Sinayobye et al., 2014.
Various evidences indicate that in HIV positive women, there is three times increase in HPV infection, tenfold higher rate of abnormal Pap smears, four to five times increase in CIN and nine times increased risk of cervical cancer. In addition CIN / cervical cancer is more severe, has extensive/larger volume of involvement of cervix, likely to involve other areas of lower genital tract as vulva/vaginal/anal region and the patients are usually 10-15 years younger than HIVnegative cases. 20-60% HIV positive women show precancerous lesion in the cervix compared to 5% in HIVnegative women. CIN among HIV-positive women was 8.3 per 100 person-years, compared with 1.8 per 100 person-years among HIV-negative women. The other facts of cervical cancer in HIV-infected women are, the rate of metastasis is increased with metastasis occurring in unusual locations (psoas muscle, clitoris, meningeal involvement), poor response to standard therapy, high recurrence and death due to disease. The recurrence/death occurs in a short interval compared to HIV-negative women of similar stage. Advanced HIV infection results in increased HPV viral load with increased frequency, severity and incidence of cervical dysplasia. Following ART treatment, there is enhanced clearance of HPV; however there is no evidence of decreased risk of cervical cancer, no regression in abnormal Pap test results but in fact due to longer life span of these patients with ART therapy, there is increased risk of CIN/cervical cancer in absence of comprehensive screening and treatment. ART has the effect on acute infection but not on advanced infection. Studies conducted in Rwanda, Kenya, South Africa; Uganda and Zambia reported prevalence of cervical pre-cancer and cancer in HIVpositive women of 24.3%, 26.7%, 66.3%, 73.0% 76.0% respectively Jean Anderson, 2012Ononogbu et al., 2013Sinayobye et al., 2014.
The proposed mechanism of action of HIV which enhances the activity of HPV in cervix is by: (1) increasing the penetration of HPV into target cells (basal cells) by disrupting epithelial tight junction by HIV tat protein and gp 120 protein, release of TNFalpha and gamma by infected cells; (2) increase expression of E and L protein, increase transcription and thus increase replication of HPV by HIV tat protein; (3) immune escape of infected cells by shift in polarisation from Th1 to Th2, abnormal cytokine expression and growth factor production. There are also evidences that HPV infection increases the likelihood of HIV infection and vice versa @(Jean Anderson, 2012; Ononogbu et al., 2013; Sinayobye et al., 2014).
The screening pattern for cervical cancer is different in HIV-positive women compared to HIV-negative women ( Table 3 ). Role of HPV test is limited to women with HIV Moyer, 2012Shaw, 2009.
Herpes Viridian group of virus
Herpes group of viruses are double-stranded DNA viruses. These are the potential candidates and interact with HPV in cervical carcinogenesis as bystanders/ cofactors. Four herpes viruses are involved; herpes simplex virus 2, CMV/ HHV 6 and EBV of alpha, beta and gamma herpes virus family respectively which has synergetic action with HPV. These four viruses share a high degree of genomic homology and, therefore, share similar oncogene potential Chan et al., 2001.
Herpes Simplex Virus 2 (HSV-2)
HSV is an encapsulated double-stranded DNA virus. It is the HSV-2 that is related with cervical cancer than HSV1. It was the first virus thought as the primary etiological factor for cervical cancer in 1960 and 1970. It is transmitted by sexual contact and proved to have synergetic role with HPV in causing CIN/cancer. Various studies of HSV-2 regarding DNA study, viral load, serological study and co-infection with HPV has been done to reveal the role of HSV-2 or synergetic action with HPV in the cervix. The HSV-2 DNA, serological positivity and viral load increased from cervicitis to CIN to cancer (both squamous cell carcinoma and adenocarcinoma). HSV-2 infection or co-infection with HPV by seroepidemiological studies is strongly higher in CIN and cervical cancer than in healthy women. However PCR results of HSV-2 DNA in cervical cancer is variable. However, still the issue is controversial and unclear ranging from no association to synergetic role with HPV in causing severe cervical lesions. Some study also states that cervical cancer may predispose to HSV-2 infection Lehtinen et al.,2002Zhao et al., 2012.
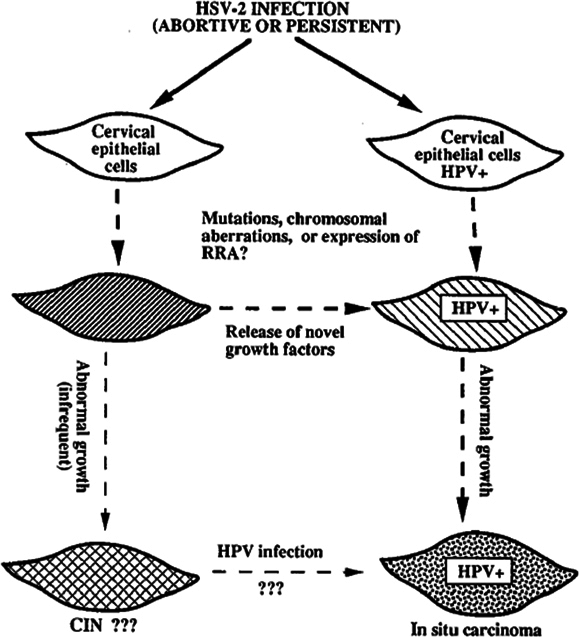
The mechanism of action of HSV-2 in causing CIN/cancer are described in the literature; “Hit and Run” hypothesis stating that the HSV-2 take part in some phase of cervical carcinogenesis and does not require retention of HSV viral genes, hence HSV-2 is not detected consistently in all cervical biopsy of CIN/cancer suggesting HSV is necessary for initial transformation of cells and not for its progression. HSV-2 infection in cervical cells with or without HPV infection leads to mutation and genetic rearrangements which has growth regulatory mechanism. In HPV-infected cells, the HSV-2 mutation is more detrimental in which HPV E6 inactivates P53 protein, causes increased abnormal proliferation of HSV-2 mutagenized epithelial cells. Thus, HSV-2 acts as a cofactor in the progression of CIN to cervical cancer by inducing DNA damage and chromosomal abnormalities in cervical epithelial cells that are latently infected with HPV. The other mechanism described are; ulcer caused by HSV-2 infection facilitates entry of HPV and reach basal cells, herpetic inflammation interfere with effective immune response against HPV infection by suppressing T-helper cell-mediated immune response, induce production of nitric acid that may result in cellular DNA damage in HPV-infected cells and increase risk of carcinogenesis, HSV-2 infection increase integration of HPV DNA into infected host cell genome, enhances replication of HPV and thus facilitates progression of HPV infection to cervical neoplasia ( Figure 2 ). HSV-2 is associated with squamous cell carcinoma, adenocarcinoma and adenosquamous carcinoma of the cervix Jones, 1995Lehtinen et al., 2002Smith et al., 2002Zhao et al., 2012. HSV-2 infection is detected by RT-PCR, PCR, in-situ hybridization techniques and serological methods of which RT-PCR is more reliable. The use of immunoglobulin A than immunoglobulin G as a marker for the presence of HSV-2 correlates with various stages of cervical cancer Jones, 1995Zhao et al., 2012.
Cytomegalovirus (CMV)
CMV infection is reported in 50-85% of the worldwide population in early adulthood. It is transmitted orally, sexually, parental and by contact with infected body fluids. There are evidences that it is associated with some human malignancies. CMV frequently infect the genital tract. Since early 1960s, CMV is documented to be associated with cervical carcinoma. Worldwide the crude frequency of CMV in cervix is 18.9% in all cervical samples and it varies in different regions as Africa 61.0%, Asia 26.9%, Europe 6.6%, North America 12.8% and Oceania 6.29%. The crude frequency of CMV worldwide in cervix in HPV-positive women is 36.5%. However, it is 52.8% in Europe and 23.8% in Asia. Hence, CMV infection frequency varied with specific population distribution and reported more in developing countries than developed. CMV is reported in all types of cervical lesions; 17.4% in normal/cervicitis, 28.0% in low-grade squamous intraepithelial lesion, 19.7% of high-grade squamous intraepithelial lesion, 44.4% in carcinoma insitu/ invasive cervical carcinoma and the overall rate is 1.58 to 61.0% in cervical lesions. A case of CMV reactivation is reported following chemo-radiation of invasive cervical carcinoma Marinho-Dias and Sousa, 2013Schlumbrecht et al.,2011.
Its role in cervical cancer is still controversial. There are evidences stating that there is no correlation between CMV and development of carcinoma cervix. On the other hand, there are also evidences stating that CMV infection has increased the risk of developing cervical cancer. The role of CMV as a co-factor for HPV or opportunistic infection is yet to be solved completely. One of the explanations is, primary infection by CMV when it persists give rise to chronic cervicitis and contributes to immunosuppression in cervix that predispose cervix to acquire HPV infection and induce HPV mediated cervical cancer. It also reactivates latent HPV infection. The other explanation is the immediate and early gene products of CMV, which transactivate the other viral/cellular genes, i.e., HPV and HIV. It also gives rise to concurrent infection by HPV Chan et al., 2001Grce et al., 2004Marinho- Dias and Sousa, 2013.
CMV can be detected by PCR, Nested PCR, RT-PCR, in-situ hybridization, immunofluorescence technique and antigenemia. PCR and RT-PCR are more reliable and sensitive Marinho-Dias and Sousa, 2013. CMV DNA is isolated in cervicovaginal secretions even when undetectable in the blood samples. Increased CMV virus infection was noted in CIN of nonpregnant women than pregnant women Grce et al.,2004.
Human Herpes Virus 6 (HHV 6)
HHV 6 infects cervical epithelial cells and alters the expression of HPV genes. HHV 6 genome is a putative oncogene that transactivates HPV. In vitro, HHV 6 enhances the expression of HPV oncoproteins E6 and E7. Hence, HHV 6 is proposed to function in a multistage carcinogenic process and causes cervical cancer by up-regulating the expression of HPV E6 and E7 oncogenes. HHV 6 is reported to enhance tumorigenicity by “Hit and Run” hypothesis. HHV 6 persists in a latent state in HPV transformed epithelial cells and gets reactivated before disease manifestation. The reactivation is increased in pregnancy and in association with HIV infection that leads to immunosuppression and increased transactivation of HPV. There are other views regarding HHV 6 that it causes progression of cervical cancer, rapid tumorigenesis and co-presence of CMV and HHV 6 DNA was significantly higher in patients with squamous intraepithelial lesion compared to controls. In 2008, Broccolo et al. found that the prevalence of HHV 6 DNA was significantly higher in high-grade squamous intraepithelial lesion. Hence, HHV 6 acts as a cofactor for HPV. However, there is no definite consensus regarding the exact mechanism of HHV 6 action in cervical carcinogenesis Chan et al., 2001Chen et al., 1994Romano et al., 1996.
Epstein-Barr Virus (EBV)
EBV is transmitted by the sexual route. EBV genome is isolated in 26% of normal cervical samples and 55% of cervical carcinoma samples by in-situ hybridization and immunofluorescence technique. EBV remains as latent infection. EBV co-infection with HPV is documented in cervical cancer cases Alibek et al., 2012. It is detected in 69% of SCC, 12.5% of CIN I, 38.9% in CIN II/III and 14% in normal cervical biopsy. Combination of HPV and EBV is detected in 67% of SCC in all histologic variants; hence EBV has a role in cervical carcinogenesis, and such cases have a bad prognosis. However, EBV has no direct role in cervical cancer. A study at Algeria showed that co-infection of EBV and HPV were detected more frequently in CIN II/III lesions and SCC than in normal and CIN I cases. EBV were absent in controls, and decline of EBV peak was noted in benign cervical lesions. This suggests a possible role of EBV in the late phase of cervical carcinogenesis as co-factor, i.e., in final invasive cancer progression. Women infected with EBV have threefold likelihood to develop cervical cancer. Still the role of EBV in cervical cancer is controversial and is a great debate Kahla et al., 2012Khenchouche et al., 2013. Another study showed HPV in 89% cases of cervical cancer and HPV with EBV in 68% cases Prayitno, 2006.
The ecto and endocervical tissue has EBV/C3d (CD 21) receptors through which EBV infects and replicates in cervical epithelium. Such cells are susceptible to other oncogenic stimuli and thus EBV has a role in malignant transformation Khenchouche et al., 2013. In a study, EBV EBNA-1 was more prevalent in cervical cancer than in benign lesions and in positive cases of EBV there was a fivefold increase in risk of HPV 16 gene integration in the host genome. Thus, EBV acts as a co-factor favouring integration of HPV DNA and thus induces cervical cancer. The other described mechanisms are, EBV-infected tumour show lymphocytic infiltration which produce viral IL10 expressed from BCRF-1 that decreases the local immunity and cause suppression of the response to HPV transformed cells; EBV contributes to the integration of HPV16 genome; EBV EBNA1 protein decreases apoptosis and DNA repair in EBVassociated lesions contributing to malignant transformation of co-transfected EBV/HPV lesions Kahla et al., 2012Khenchouche et al., 2013. EBV activates the growth-promoting pathway that are normally triggered by T cell-derived signal Alibek et al., 2012. Expression rates of EBER-1 mRNA, LMP-1 and EBNA-2 were significantly higher in invasive cervical cancer and CIN than normal cervical tissue suggesting EBV infection is involved in the development of cervical cancer Chhetri, 2010.
EBV can be detected by PCR, RT-PCR, IHC, immunoblotting (Western blotting, Southern blotting) by detecting EBV DNA, BERF-1, EBNA-1 and LMP-1. PCR is more sensitive Khenchouche et al., 2013.
Adeno-Associated Virus (AAV)
AAV is single-stranded DNA virus, a human helper virus dependent Parvovirus. It resided in the genital tract and transmitted sexually. It has the ability to suppress the oncogenic phenotype of variety of viruses. It prevents the replication of HPVs and thus prevents the development of CIN in HPV-infected patients in vitro. However in-vivo studies in women with HPV infection or CIN showed inconsistent results regarding its role in cervical carcinogenesis. It reactivates latent HPV infection. Increased AAV2 virus was noted in pregnant women than non-pregnant women indicating the reactivation of latent infection due to hormonal changes and slight immunosuppression. Hence, it is protective against the progression of CIN in pregnancy Grce et al., 2004.
Hepatitis C Virus (HCV)
Hepatitis C virus is a single-stranded RNA virus reported to be isolated from the malignant tissue of the cervix that caused chronic hepatitis in a 54-year woman. Hence, they concluded that HCV might have a direct role in the development and pathogenesis of cervical cancer Sasagawa et al., 2000.
Conclusion
Better understanding of viral aetiology and its pathogenesis helps in primary/secondary prevention, better diagnosis of in early stages of cervical cancer and hopefully helps in therapy.
Abbreviations
AAV: Adeno-associated Virus; ALTS: ASC-US /LSIL Triage Study; ART: Anti-Retroviral Therapy; CD: Cluster Differentiation; CIN: Cervical Intraepithelial Neoplasia; CMV: Cytomegalovirus; DNA: Deoxyribonucleic acid; E1 to E7: Early genes / Protein; EBER: EBV encoded small nuclear RNA; EBNA: Epstein Barr Virus nuclear antigen; EBV: Epstein Barr Virus; HC2: Hybrid Capture 2; HCV: Hepatitis C Virus; HHV 6: Human Herpes Virus 6; HIV: Human Immunodeficiency Virus; HPV: Human Papilloma Virus; HR HPV: High Risk Human Papilloma Virus; HSV 2: Human Simplex Virus 2; L1 / L2: Late capsid genes / Protein; LMP: Latent membrane protein; NPV: Negative Predictive Value; PCR: Polymerase chain reaction; PPV: Positive Predictive Value; RNA: Ribonucleic acid; RTPCR: Real Time PCR; SCC: Squamous Cell Carcinoma; Th: T helper (Lymphocytes) cells; TNF: Tumour Necrosis Factor; USA FDA: United States of America – Food & Drug Administration; VLP: Virus like particles
References
-
A.L.P.
Abreu,
R.P.
Souza,
F.
Gimenes,
M.E.L.
Consolaro.
A review of methods for detect human Papillomavirus infection. Virology Journal.
2012;
9
:
262-262
.
-
K.
Alibek,
N.
Karatayeva,
I.
Bekniyazov.
The role of infectious agents in urogenital cancers. Infectious agents and cancer.
2012;
7
:
3-5
.
-
B.S.
Apgar,
A.L.
Kittendorf,
C.M.
Bettcher,
J.
Wong,
A.J.
Kaufman.
Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. American family physician.
2009;
80
:
147-155
.
-
P.K.
Chan,
M.Y.
Chan,
W.W.
Li,
D.P.
Chan,
J.L.
Cheung,
A.F.
Cheng.
Association of human beta-herpesviruses with the development of cervical cancer: bystanders or cofactors. Journal of clinical pathology.
2001;
54
:
48-53
.
-
M.
Chen,
N.
Popescu,
C.
Woodworth,
Z.
Berneman,
M.
Corbellino,
P.
Lusso,
D.V.
Ablashi,
J.A.
DiPaolo.
Human herpesvirus 6 infects cervical epithelial cells and transactivates human papillomavirus gene expression. Journal of virology.
1994;
68
:
1173-1178
.
-
M.
Chhetri.
Chronic Hepatitis C Virus Infection and Carcinoma Cervix - Report of a Case and Brief Review of Literature. Apollo Medicine.
2010;
7
:
61-63
.
-
M.
Grce,
K.
Husnjak,
M.
Matovina,
N.
Milutin,
L.
Magdic,
O.
Husnjak,
K.
Pavelic.
Human papillomavirus, cytomegalovirus, and adeno-associated virus infections in pregnant and nonpregnant women with cervical intraepithelial neoplasia. J Clin Microbiol.
2004;
42
:
1341-1344
.
-
http://www.virology.uct.ac.za/teachhpv.html.
.
-
E.L.
Jean Anderson,
Sharon Kibwana Anjanique Lu
Harshad Sanghvi.
Cervical Cancer Screening and Prevention for HIV-Infected Women in the Developing World. In Cancer Prevention - From Mechanisms to Translational Benefits, A. Georgakilas, ed.,.
2012;
:
231-260
.
-
X.W.
Jin,
L.
Lipold,
M.
McKenzie,
A.
Sikon.
Cervical cancer screening: what's new and what's coming?. Cleveland Clinic journal of medicine.
2013;
80
:
153-160
.
-
C.
Jones.
Cervical cancer: is herpes simplex virus type II a cofactor?. Clinical microbiology reviews.
1995;
8
:
549-556
.
-
S.
Kahla,
S.
Oueslati,
M.
Achour,
L.
Kochbati,
M.B.
Chanoufi,
M.
Maalej,
R.
Oueslati.
Correlation between ebv coinfection and HPV16 genome integrity in Tunisian cervical cancer patients. Brazilian Journal of Microbiology.
2012;
43
:
744-753
.
-
R.
Kalyani,
S.
Das,
M.S.
Bindra Singh,
H.
Kumar.
Cancer profile in the Department of Pathology of Sri Devaraj Urs Medical College, Kolar: a ten years study. Indian J Cancer.
2010;
47
:
160-165
.
-
A.
Khenchouche,
N.
Sadouki,
A.
Boudriche,
K.
Houali,
A.
Graba,
T.
Ooka,
A.
Bouguermouh.
Human Papillomavirus and Epstein-Barr virus co-infection in Cervical Carcinoma in Algerian women. Virology Journal.
2013;
10
:
340-340
.
-
K.S.J.
Krishnakumar Duraisamy,
Bose
Jagathesh Chandra.
Methods of Detecting Cervical Cancer. Advance in Biological Research.
2011;
5
:
226-232
.
-
M.
Lehtinen,
P.
Koskela,
E.
Jellum,
A.
Bloigu,
T.
Anttila,
G.
Hallmans,
T.
Luukkaala,
S.
Thoresen,
L.
Youngman,
J.
Dillner.
Herpes simplex virus and risk of cervical cancer: a longitudinal, nested case-control study in the nordic countries. American journal of epidemiology.
2002;
156
:
687-692
.
-
J.
Marinho-Dias,
H.
Sousa.
Cytomegalovirus infection and cervical cancer: from past doubts to present questions. Acta medica portuguesa.
2013;
26
:
154-160
.
-
V.A.
Moyer.
Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med.
2012;
156
:
880-891
.
-
NobelPrize.
The Nobel prize in Physiology or medicine. The Nobel prize in Physiology or medicine.
2008
.
-
U.
Ononogbu,
M.
Almujtaba,
F.
Modibbo,
I.
Lawal,
R.
Offiong,
O.
Olaniyan,
P.
Dakum,
D.
Spiegelman,
W.
Blattner,
C.
Adebamowo.
Cervical cancer risk factors among HIV-infected Nigerian women. BMC Public Health.
2013;
13
:
58-2
.
-
A.
Prayitno.
Cervical cancer with human papilloma virus and Epstein Barr virus positive. Journal of carcinogenesis.
2006;
5
:
1-3
.
-
N.
Romano,
F.M.
Romano,
E.
Viviano,
F.
Vitale,
M.R.
Villafrate,
A.M.
Perna,
F.
Bonura,
R.
Guttadauro.
Rare association of human herpesvirus 6 DNA with human papillomavirus DNA in cervical smears of women with normal and abnormal cytologies. J Clin Microbiol.
1996;
34
:
1589-1591
.
-
T.
Sasagawa,
M.
Shimakage,
M.
Nakamura,
J.
Sakaike,
H.
Ishikawa,
M.
Inoue.
Epstein-Barr virus (EBV) genes expression in cervical intraepithelial neoplasia and invasive cervical cancer: a comparative study with human papillomavirus (HPV) infection. Hum Pathol.
2000;
31
:
318-326
.
-
M.
Schlumbrecht,
K.
Grimes,
J.
Brown.
Cytomegalovirus reactivation following chemoradiation for invasive cervical carcinoma. Gynecologic oncology case reports.
2011;
1
:
22-23
.
-
F.
Shaw.
CDC Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-infected adults and adolescents. MMWR.
2009;
58
:
68-75
.
-
J.
Sinayobye,
M.
Sklar,
D.
Hoover,
Q.
Shi,
J.
Dusingize,
M.
Cohen,
E.
Mutimura,
B.
Asiimwe-Kateera,
P.
Castle,
H.
Strickler.
Prevalence and risk factors for High-Risk Human Papillomavirus (hrHPV) infection among HIV-infected and Uninfected Rwandan women: implications for hrHPV-based screening in Rwanda. Infectious agents and cancer.
2014;
9
:
4-0
.
-
J.S.
Smith,
R.
Herrero,
C.
Bosetti,
N.
Munoz,
F.X.
Bosch,
J.
Eluf- Neto,
X.
Castellsague,
C.J.
Meijer,
A.J.
Van den Brule,
S.
Franceschi.
Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst.
2002;
94
:
1604-1613
.
-
L.L.
Villa,
L.
Denny.
CHAPTER 7 Methods for detection of HPV infection and its clinical utility. International Journal of Gynecology and Obstetrics.
2006;
94
:
S71-S80
.
-
T.C. Jr.
Wright,
L.S.
Massad,
C.J.
Dunton,
M.
Spitzer,
E.J.
Wilkinson,
D.
Solomon.
2006 consensus guidelines for the management of women with abnormal cervical screening tests. Journal of lower genital tract disease.
2007;
11
:
201-222
.
-
Y.
Zhao,
X.
Cao,
Y.
Zheng,
J.
Tang,
W.
Cai,
H.
Wang,
Y.
Gao,
Y.
Wang.
Relationship between cervical disease and infection with human papillomavirus types 16 and 18, and herpes simplex virus 1 and 2. Journal of medical virology.
2012;
84
:
1920-1927
.
Comments
Downloads
Article Details
Volume & Issue : Vol 2 No 03 (2015)
Page No.: 220-230
Published on: 2015-03-10
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 9287 times
- Download PDF downloaded - 1873 times
- View Article downloaded - 6 times
 Biomedpress
Biomedpress