OPTIMIZATION OF CULTURE MEDIUM FOR THE ISOLATION AND PROPAGATION OF HUMAN BREAST CANCER CELLS FROM PRIMARY TUMOUR BIOPSIES
Abstract
Breast cancer cells from patients hold an important role in antigen production for immunotherapy, drug testing, and cancer stem cell studies. To date, although many studies have been conducted to develop protocols for the isolation and culture of breast cancer cells from tumour biopsies, the efficiencies of these protocols remain low. This study aimed to identify a suitable medium for the isolation and propagation of primary breast cancer cells from breast tumour biopsies. Breast tumour biopsies were obtained from hospitals after all patients had given their written informed consent and were cultured according to the expanding tumour method in 3 different media: DMEM/F12 (Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12) supplemented with 10% FBS (Fetal bovine serum) and 1% antibiotic-antimycotic (Medium D); Medium 171 supplemented with 1X MEGS (Mammary Epithelial Growth Supplement) and 1% antibiotic-antimycotic (Medium M); or a 1:1 mixture of Medium D and Medium M (Medium DB). The cell culture efficiency was evaluated by several criteria, including the time of cell appearance, cell morphology, capability of proliferation, cell surface marker expression, ALDH (Aldehyde dehydrogenases) activity, karyotype, and tumour formation capacity in immune-deficient mice. Notably, primary cancer cells cultured in Medium DB showed a high expression of breast cancer stem cell surface markers (including CD44+CD24- and CD49f+), low expression of stromal cell surface markers (CD90), high ALDH activity, an abnormal karyotype, and high tumour formation capacity in immune-deficient mice. These findings suggested that Medium DB was suitable to support the survival and proliferation of primary breast cancer cells as well as to enrich breast cancer stem cells.
Introduction
The successful primary culture of cancer cells from breast tumours has great significance for the creation of an original cell source to study breast cancer cells (BCC) biology and for the development of therapeutic strategies. Although many studies on cancer cell biology have been conducted using cancer cell lines Gillet et al., 2011Gillet et al., 2013Lacroix and Leclercq, 2004Neve et al., 2006, recent reports have highlighted that those cancer cell lines do not, for various reasons, consistently display original cell characteristics Keller et al., 2010. Therefore, to ensure that a chosen cell culture model accurately reflects the biological characteristics of the cells of interest, the use of primary cancer cells is essential.
Numerous primary culture studies are underway to identify optimal conditions, with regard to efficiency and duration, for the acquisition of BCCs. While there are many factors that influence the primary culture process, the culture medium is the key parameter. In studies on HBC cells, culture conditions were initially adopted from a number of similar studies on normal HME cells that had identified important factors determining their in vitro survival Hammond et al., 1984Stampfer et al., 1981aStampfer et al., 1981b. Regrettably, medium components for the propagation of primary human breast cells (HBC) have not yet been determined, as the biology of this cell type remains largely unclear. Stampfer et al. Stampfer, 1982Stampfer and Bartley, 1985Stampfer et al., 1993 developed a variety of culture media for the growth of human mammary epithelial (HME) cells; the original medium consisted of several undefined components, but was later refined to a hormone- and growth factorsupplemented medium that supports proliferation of HME cells over many in vitro passages. Band and Sager Band and Sager, 1989 showed that it was useful to propagate HME cells extensively in a growth factor- and hormone-supplemented medium that also contained serum and pituitary extract. Subsequently, Petersen and Van Deurs Petersen and van Deurs, 1987 and Ethier et al. Ethier et al., 1991 reported the growth of normal HME cells in serum-free media in the absence of pituitary extract or serum. Although these culture media promoted the growth of normal mammary epithelial cells, very few of them supported the growth of BCCs Bartek et al.,1985Taylor- Papadimitriou et al., 1989. Thus, the culture conditions that are conducive to the rapid proliferation of normal HME cells over many in vitro passages hardly support the growth of BCCs Wolman et al., 1985.
The use of a serum-free medium for the cultivation of normal HME cells circumvents a number of problems that are mainly related to the instability of serum Hammond et al.,1984Smith et al., 1981. However, in comparison with serum-containing medium, serum-free medium also entails some disadvantages such as the need for a complex mixture of highly pure medium components and a reduced cell proliferation rate. Mammary tumour cell lines have been isolated and grown in standard medium (e.g. Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12, DMEM/F12) supplemented with 10% foetal bovine serum (FBS) Engel and Young, 1978Smith et al., 1987Soule et al., 1973. Serum contains growth factors, which promote cell proliferation, as well as adhesion factors and antitrypsin activity, which promote cell attachment. However, it has recently been accepted that tumours consist of highly heterogeneous cell populations with respect to cellular morphology, proliferative potential, genetic lesions, and treatment response Bomken et al., 2010Marusyk and Polyak, 2010. The isolation of cells from breast tumours may give rise to several different cell types; normal counterparts from which the neoplastic cells arise, such as connective-tissue fibroblasts, infiltrating immune cells, vascular endothelial cells, and smooth muscle cells, as well as other elements of the normal tissue can all survive explantation Sung et al., 2007Weber and Kuo, 2012Yu et al., 2011. Therefore, breast cancer epithelial cells in tumour biopsies are irrevocably overgrown by fibroblasts in a medium supplemented with high serum concentrations.
In any case, the success rate of a BCC culture is increased greatly by using selective media that enrich the population of BCCs, but prevent the rapid and extensive growth of normal cells, including stromal cells and normal HME cells. Ethier et al. found that the addition of 5% FBS to medium supplemented with insulin, hydrocortisone, EGF, cholera toxin, and progesterone stimulated rapid proliferation of breast cancer epithelial-like cells Ethier et al., 1993. They also found that a relatively simple medium, only supplemented with 5% FBS, insulin, and hydrocortisone, resulted in the slow emergence of BCCs that ultimately gave rise to BCC lines Ethier et al., 1993.
To date, the essential characteristics of primary breast cancer cells are still a matter of debate. In particular, recent studies have shown that solid breast tumours harbour a cell population with stem cell characteristics, which is responsible for the formation and maintenance of tumours, development of metastases, and, eventually, patient mortality. These cells are known as cancer stem cells Al-Hajj et al., 2003Clarke, 2005. In this study, we aimed to develop a standardized protocol for the isolation and propagation of HBC cells from primary tumour biopsies that a) ensures that the isolated primary cells include breast cancer stem cells and b) minimizes contamination with other cells.
Material and Methods
Culture medium
Medium D: DMEM/F12 (1:1, v/v) supplemented with 10% FBS and 1% antibiotics/antimycotics (100X) (all bought from GeneWorld, HCM, Vietnam). Medium M: Medium 171 supplemented with 1% MEGS (100X), and 1% antibiotics/antimycotics (all bought from Life Techonologies, Carlsbad, CA). Medium DB: mixed by medium D and medium M (1:1, v/v).
Karyotyping reagents
Hypotonic solution: KCl 0.075 M and sodium citrate 0.8% (1:1, v/v); Fix solution (Carnoy’s solution): Methanol and glacial acetic acid (1:1, v/v).
Animals
Immunodeficient athymic nude mouse 7-8 weeks old (NU(NCr)-Foxn1nu) were purchased from Charles Rivers (Sulzfeld, Germany), kept under pathogen-free conditions, and handled in accordance with the institutional recommendations for experimentation.
Primary culture of HBC cells from malignant tumour specimens
Ten tumour biopsies, all from different patients, were collected after obtaining the patients’ consent and the approval from the ethics committee and were transported to the laboratory on ice. Excess adipose tissue was pared off the tumour samples, following which the samples were sliced into small fragments (approximately 1-2 mm2) by using a scalpel, while care was taken not to tear the tissue. Twelve of these fragments were seeded per T25 tissue culture flask. Groups of 3 flasks then received either Medium D, Medium M, or Medium DB for cultivation of the cells. The flasks were incubated at 37°C with 5% CO2 and monitored daily to record the time of cell appearance, the cell morphology, as well as the number of tissue fragments with cell migration. The medium was replaced every 3 days.
Primary cancer cell phenotyping by flow cytometry
Primary cells were analysed for surface marker expression by flow cytometry. CD44, CD24, and CD49f were recorded to determine the percentage ratio of breast cancer stem cells (BCSCs), and CD90 was used to monitor any fibroblast contamination. After 4 weeks of continuous culture, primary cells were detached using 0.25% trypsin/EDTA (GeneWorld, Ho Chi Minh, Vietnam). A total of 1 × 106 cells were stained with anti-CD44-APC (allophycocyanin) and anti-CD24-FITC (fluorescein isothiocyanate), anti-CD49f-FITC, or anti-CD90-FITC. Then, cells were washed twice with sheath fluid, and subsequently analysed by a FACSCalibur flow cytometry instrument (BD Biosciences, San Jose, CA). Ten thousand events were acquired in triplicate and analysed using CellQuest Pro software (BD Biosciences, San Jose, CA).
ALDEFLUOR stem cell identification assay
Following 4 weeks of continuous culture, primary cells were harvested and subjected to the ALDEFLU- OR assay, according to the manufacturer’s instructions (Stemcell Technologies, Vancouver, BC, Canada). Briefly, the primary cell suspension was divided into 2 tubes per sample, with 1 × 106 cells per tube. Tube 1 served as the control and received ALDH reagent, followed by ALDH DEAB reagent, whereas tube 2 served as sample and received only ALDH reagent. The cells were stained with 5 μl of these reagents for 30 min in the cell incubator, and then washed twice with sheath fluid. Finally, the samples were analysed by a FACSCalibur flow cytometry instrument (BD Biosciences, San Jose, CA). Ten thousand events were acquired in triplicate and analysed by CellQuest Pro software (BD Biosciences, San Jose, CA).
Karyotyping
Well-proliferating primary cells were treated with colcemid at a concentration of 0.10 μg/ml for 3 hours. Primary cells were harvested, and then used for karyotyping by following a previously published protocol. Briefly, the single cell suspension was incubated in hypotonic solution for 30 min at 37°C, and then fixed at least 3 times in Carnoy’s solution, which included an overnight fixative step. The fixed cell suspension was dropped on well-prepared slides and stained according to the G-Banding protocol. Sets of chromosomes were analysed using Ikaros software (MetaSystems, Altlussheim, Germany).
Tumourigenesis assay
Five experimental groups were defined to evaluate the effects of the 3 different kinds of culture medium on the tumourigenic potential of primary BCCs. Each group comprised 3 mice. In group 1, primary cells cultured in Medium D were injected into the mammary fat pad at cell densities of 106, 105, 104, and 103 cells per 100 μl PBS by using the right and left sides of the same mouse. Similarly, the mice in group 2 and 3 were injected with primary cells cultured in Medium M and Medium DB, respectively, at the same cell densities. Group 4 comprised control mice that were injected with cells of the MCF-7 BCC line (ATCC, Manassas, VA), whereas group 5 comprised control mice injected with cells of the MDA-MB-231 BCC line (ATCC, Manassas, VA), again at the aforementioned cell densities. Two weeks post-injection, the tumour size was measured and calculated using the equation: (length × width2)/2.
Results
Primary culture of HBC cells from malignant tumour specimens
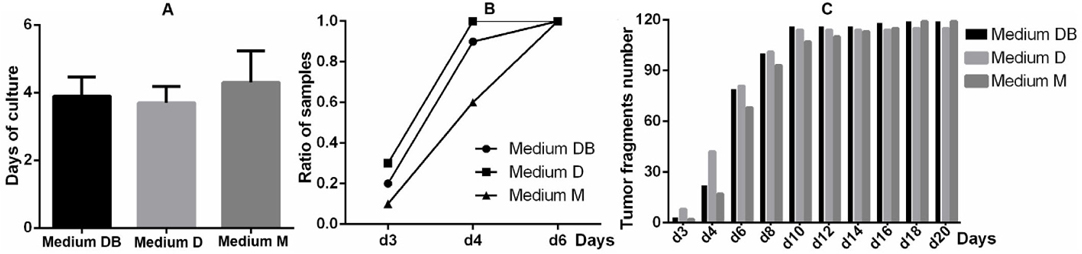
For all 10 breast cancer biopsies, primary cells were observed to spread out from the tumour fragments. Primary cells began to migrate from tumours around day 4, with the earliest migrating cells being recorded at day 3 ( Figure 1A ). The percentage ratio of samples displaying cell migration was highest when cultured in Medium D (3/10 samples at day 3 and 10/10 at day 4), followed by samples cultivated in Medium DB (3/10 samples at day 3, 9/10 at day 4, and 10/10 at day 6), and Medium M (1/10 samples at day 3, 6/10 at day 4, and 10/10 at day 6) ( Figure 1B ). Consistently, at the early stages of the culture period, the largest proportion of successfully cultured tumour fragments was observed for Medium D, followed by Medium DB, and finally Medium M. However, at the late stages of the culture period, this trend was no longer significant ( Figure 1C ).
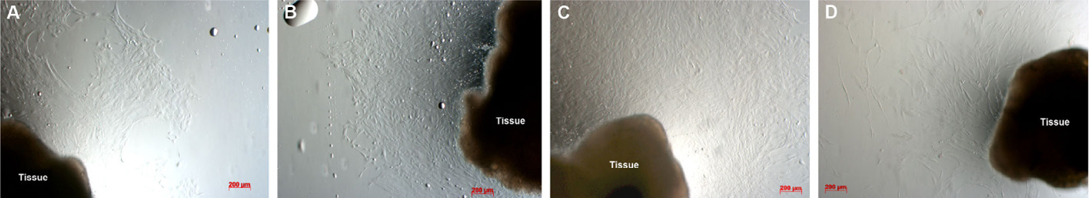
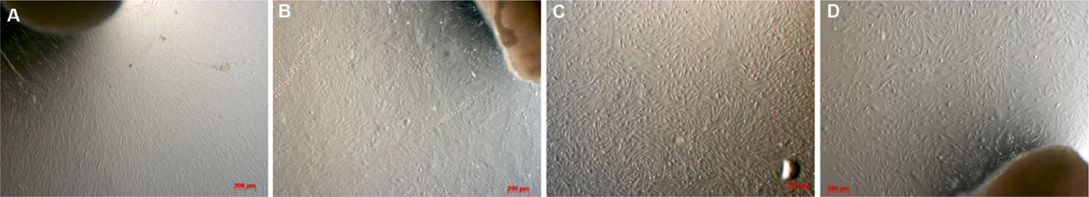
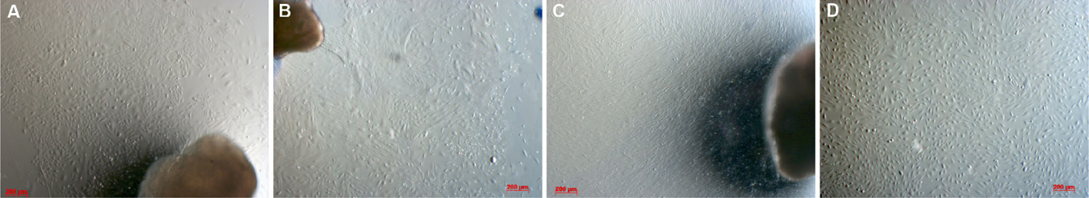
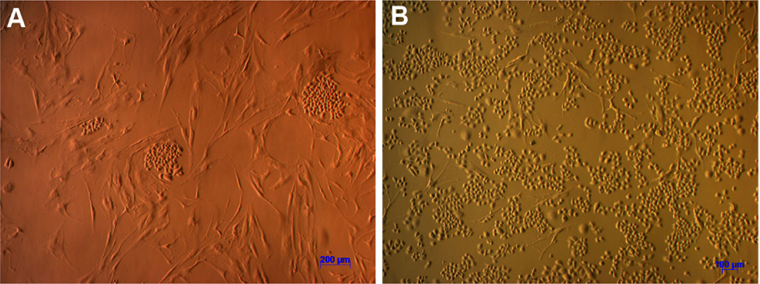
When investigating cell morphology, it was noted that samples cultured in Medium D gave rise to primary cells that uniformly exhibited a stromal-like, elongated shape, containing a small nucleus, thus resembling fibroblasts ( Figure 2 ). In contrast, almost all samples cultured in either Medium M or Medium DB formed 2 differently shaped kinds of primary cells: epitheliallike cells with a bean-like shape, having a large nucleus and mesenchymal-like cells with an elongated shape, having a small nucleus ( Figure 3 and Figure 4 , respectively).
During the 4-week cultivation period, it was visually observed that the samples cultured in Medium D, compared with those cultured in Medium DB and Medium M, showed a markedly reduced proliferation rate. While primary cells cultured in Medium D were the earliest to migrate from tumour fragments, they proliferated slowly and soon stopped dividing. This is why some of these samples did not provide a sufficient number of cells for further experiments. In contrast, primary cells cultured in either Medium DB or Medium M proliferated rapidly, and almost all of these samples provided a sufficient number of cells for subsequent experiments.
Primary cancer cell surface marker analysis
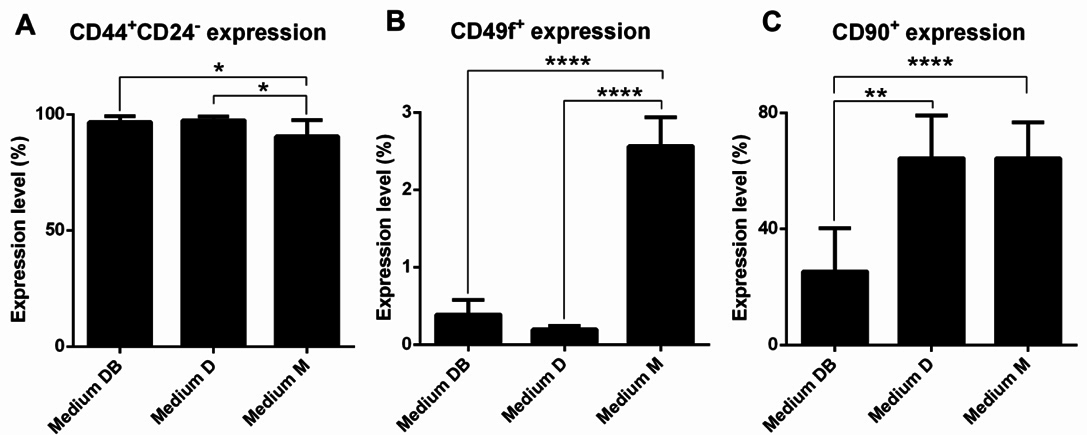
Primary cells were analysed for the surface markers CD44, CD24, and CD49f to identify the proportion of BCSCs among the primary BCC populations. Furthermore, the proportion of stromal cells present in culture was determined by monitoring the expression of CD90. Interestingly, nearly all primary cells were positive for CD44 and negative or weakly positive for CD24. This population comprised 96.69% ± 2.57%, 97.49% ± 1.512%, and 90.51% ± 7.11% of primary cells cultured in Medium DB, Medium D, and Medium M, respectively ( Figure 5A ). The proportion of CD44+CD24-/lowcells in Medium DB and Medium D was significantly higher than that in Medium M (p < 0.05).
Further, all primary cell samples harboured a small population of cells that was positive for CD49f. The proportion of CD49f+ cells amounted to 0.39% ± 0.19%, 0.20% ± 0.04%, and 2.57% ± 0.37% of the primary cells grown in Medium DB, Medium D, and Medium M, respectively ( Figure 5B ). Thus, the proportion of the CD49f+ cell population in Medium M was significantly higher than that in Medium DB and Medium D (p < 0.0001).
Moreover, it was found that the CD90+cell population comprised 25.28% ± 14.86%, 64.39% ± 14.81%, and 64.28% ± 12.39% of primary cells cultured in Medium DB, Medium D, and Medium M, respectively ( Figure 5C ), indicating that the CD90+cell population in Medium DB was significantly smaller than that in Medium D (p < 0.005) and Medium M (p < 0.0001).
ALDEFLUOR stem cell identification assay
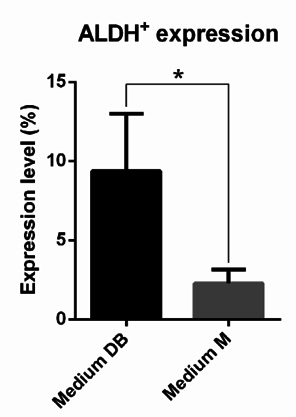
All samples harboured a small population of cells that displayed aldehyde dehydrogenase activity. The cell population testing positive for ALDH activity represented 9.35% ± 3.64% and 2.28% ± 0.88% of the primary cells grown in Medium DB and Medium M, respectively ( Figure 6 ). Thus, the size of the ALDH+cell population in Medium DB was larger than that in Medium M (p < 0.05). Note that the number of cells derived from primary culture in Medium D was insufficient for the ALDEFLUOR assay.
Karyotyping
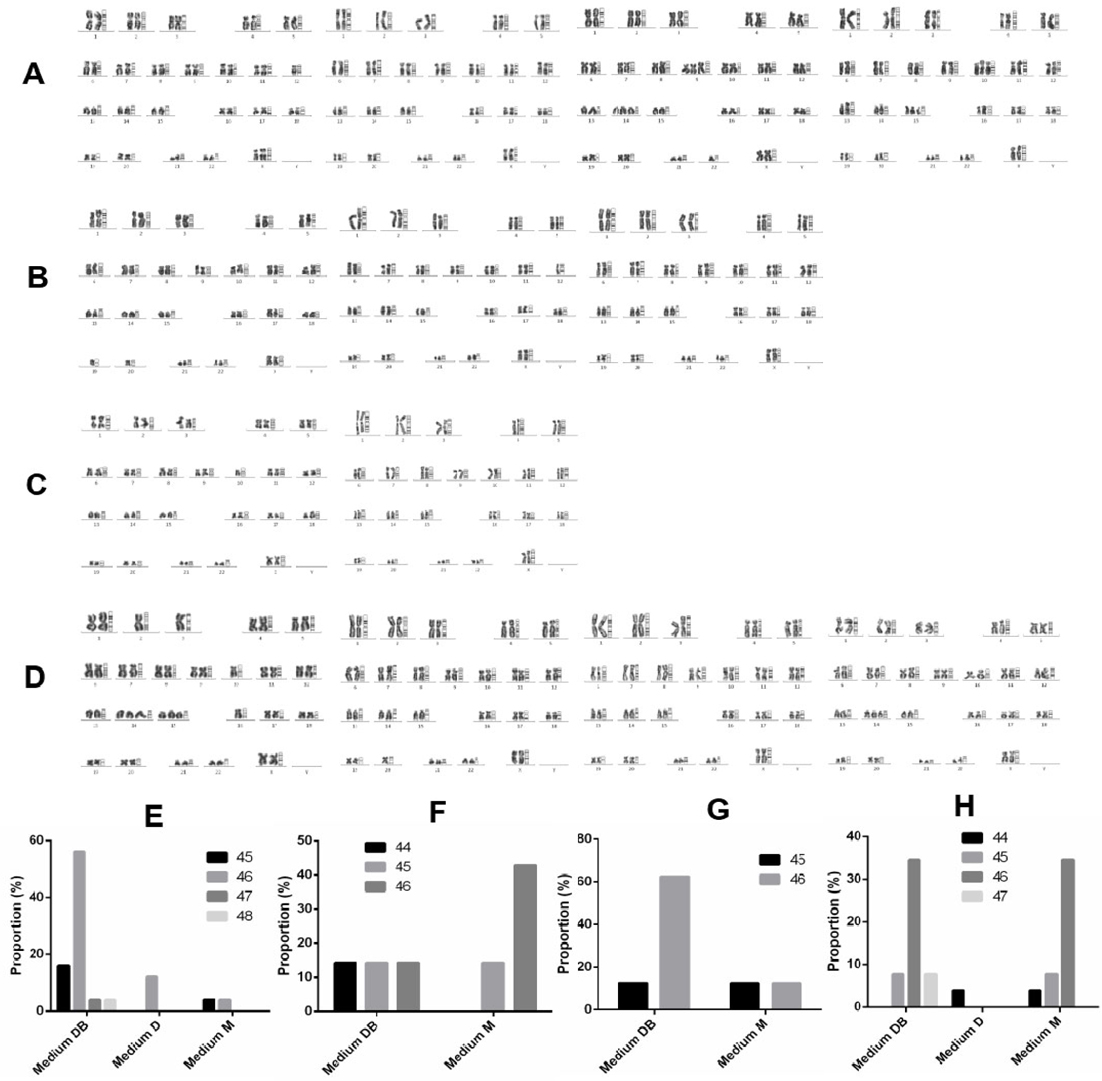
Four rapidly proliferating samples were subjected to karyotyping to determine which culture medium supported the growth of cancer cells, as defined by abnormal chromosome number. The chromosome numbers of primary cells derived from sample 1 ranged between 45 and 48 ( Figure 7A,E ). Specifically, cells cultured in Medium D uniformly contained 46 chromosomes, whereas chromosome numbers ranged between 45 and 46 in cells grown in Medium M and between 45 and 48 in cells cultivated in Medium DB ( Figure 7A,E ).
Primary cells derived from sample 2 contained between 44 and 46 chromosomes ( Figure 7B,F ). Here, cells cultured in Medium M displayed between 45 and 46 chromosomes, while Medium DB supported the growth of cells with a wider range of chromosome numbers, i.e. between 44 and 46 ( Figure 7B,F ). The number of cells derived from the primary culture in Medium D was insufficient for karyotyping. In primary cells derived from sample 3, chromosome numbers ranged between 44 and 46 ( Figure 7C,G ). Cells cultured in either Medium M or Medium DB contained between 44 and 46 chromosomes ( Figure 7C,G ). Again, the number of cells derived from the primary culture in Medium D was insufficient for karyotyping. Primary cells derived from sample 6 displayed chromosome numbers between 44 and 47 ( Figure 7D,H ). Specifically, cells cultured in Medium D uniformly harboured 44 chromosomes, whereas chromosome numbers ranged between 44 and 46 in cells cultured in Medium M, and between 45 and 47 in cells cultured in Medium DB ( Figure 7D,H ).
Tumourigenesis assay
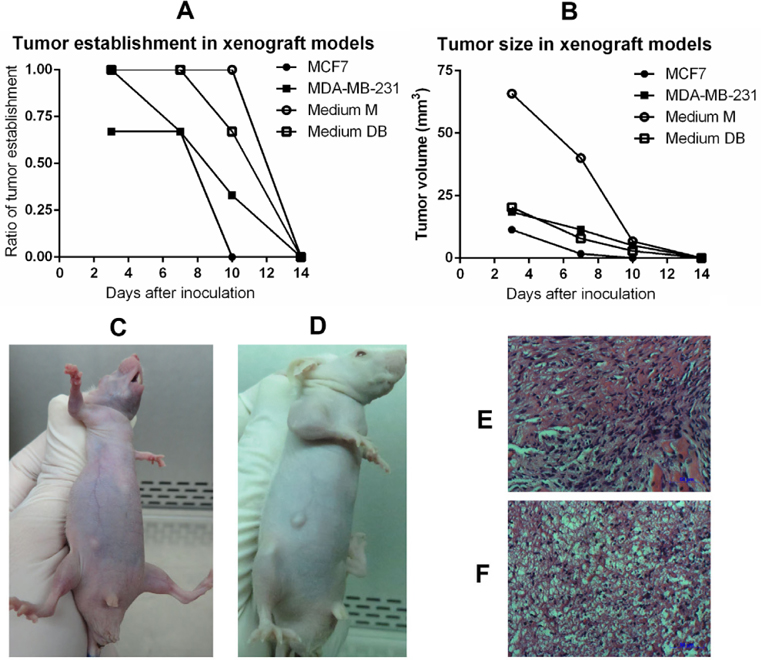
In the tumourigenesis assay, the injection of 103, 104, and 105 primary cells failed to cause tumour growth in immunodeficient mice, regardless of whether they were cultured in Medium M or Medium DB. The same observations were made for the 2 positive controls that had been injected with either MCF-7 or MDA-MB-231 BCC line. In response to an injection of 106 primary cells, all mice established a tumour and maintained it for 2 weeks. The tumourigenicity of primary cells was also higher than that of either BCC line, i.e. MCF-7 or MDA-MB-231 ( Figure 8 ).
To confirm the histopathology of tumours, 10 μm tumour sections were stained with haematoxylin-eosin (HE). As shown in Figure 8E,F , tumours exhibited cancer cells with large nuclei; the tumours were established from primary cells cultured in Medium DB and Medium M.
Mesenchymal-epithelial transition (MET) and establishment of BCC lines
Together, these results indicated that Medium DB surpassed the other media in supporting the growth of BCCs. Therefore, Medium DB was chosen to culture and maintain cells derived from malignant breast tumours. Primary cells also migrated from tumour fragments after 4-5 days of culture. In almost all samples, epithelial-like cells and mesenchymal-like cells appeared simultaneously; however, all cells transformed in to mesenchymal-like shape after 1 month of continuous culture, including cells with epithelial phenotype previously. Interestingly, following long-term culture (approximately 6 months), mesenchymal-shape cancer cells were observed to undergo back to the process of MET in culture.
Initially, some mesenchymal-like cells shrunk in size and adopted an epithelial shape. Soon, neighbouring cells also displayed this phenomenon ( Figure 9A ). This process continued and led to the formation of colonies of epithelial cells that spread over the entire surface of the culture, until all cells in the culture flask had adopted an epithelial shape ( Figure 9B ). The described process occurred naturally without the use of any stimulants, apart from the regular replacement of culture medium. These cells then proliferated rapidly and formed cell lines. Hence, the BCC line described herein was successfully developed from malignant human breast tumours via the explant culture method. These cells exhibit all typical characteristics of BCC lines and exhibit particular properties that they share with the original tumour.
Discussion
Breast tumours contain a combination of various kinds of cells, including normal epithelial cells, stromal cells, breast cancer cells, and breast cancer stem cells. A suitable protocol for the isolation of BCCs must not only provide for high cell growth efficiency, but also for the establishment of cells exhibiting BCC properties. From the information gathered from previous studies investigating single cell culture versus explant tissue culture, this study employed expanding tissue culture (data not shown). In this method, the culture medium is the most decisive factor in the outgrowth of cells from tumour fragments, as well as in the types of cells obtained. Based on existing literature reports, 3 different kinds of media were chosen for use in this study. M171 medium supplemented with MEGS (Medium M) is a serum-free medium that supports the proliferation of normal human epithelial mammary cells. In contrast, DMEM/F12 medium supplemented with 10% FBS (Medium D) is a serumcontaining medium that supports the proliferation of routine human BCC lines. For the purpose of this study, these 2 kinds of medium were mixed in a ratio of 1:1 to produce a third medium (Medium DB). Thus, Medium DB contained 50% of each of the components of Medium D and Medium M, and the serum concentration was similarly reduced to 5%.
As detailed in the Results section, Medium D was not suitable for the isolation of BCCs. Although in this medium the cells migrated more rapidly than in Medium M and Medium DB, they also showed a relatively slow mitosis rate and therefore reduced proliferation. Hence, in nearly all samples, cells grown in Medium D were not sufficiently high in number for use in further evaluation. In contrast to Medium D, primary cells cultured in Medium M proliferated rapidly, and the majority of samples cultured in Medium M provided enough cells for additional experiments. This observation can be explained by the fact that Medium M contained a pool of hormones and growth factors such as hydrocortisone, EGF, and insulin. These factors are beneficial for the survival and proliferation of breast tissue-derived cells. Primary cells cultured in Medium DB proliferated most rapidly, such that all samples provided cells that were sufficiently high in number for use in further experiments. These results are likely due to the components of Medium DB, which included growth factors and hormones from Medium M, as well as serum from Medium D. However, it should be highlighted that the serum concentration is reduced in comparison to common serum levels, and that this appears to be beneficial with regard to the elimination of stromal cells. Therefore, using Medium DB for primary tumour cell culture results in the rapid proliferation of primary cell populations.
Next, the existence of a CD44+CD24- population was evaluated by flow cytometry in all primary cell cultures. Nearly all primary cells grown in any of the 3 culture media tested positive for CD44 and negative for CD24, with the highest percentage of CD44+CD24-cells occurring in Medium D and Medium DB. In a previous study by Al-Hajj et al. (2003), primary cells contained a sub-population of CD44+CD24- cells with high tumourigenicity Al-Hajj et al., 2003. However, the possibility that the primary cells expressing CD44+CD24- were not all BCSCs appears likely Ghebeh et al., 2013Mannello, 2013. In a recent study, Ghebeh et al. (2013) clearly demonstrated that both normal breast tissue and breast cancer tissue harboured CD44+CD24- cells Ghebeh et al., 2013. They also suggested that BCSCs would be enriched in the CD44+CD24-cells if they were combined with the CD49f+phenotype Ghebeh et al., 2013. CD49f was also determined as a marker of BCSCs in previous studies Meyer et al., 2010Yu et al., 2012. Therefore, in the next experiment, the existence of a CD49f+cell population in the primary cells was evaluated. The results showed that primary cultures in Medium M contained the highest percentage of CD49f+cells, followed by primary cultures in Medium DB and Medium D. Hence, Medium M efficiently supported the growth of cells with the CD49f+phenotype; however, compared with Medium D and Medium DB, Medium M did not support the growth of CD44+CD24- cells. Medium DB excellently promoted the growth of CD44+CD24- cells, but little impact on the proliferation of CD49f+cells.
However, Medium D and Medium M also supported stromal cell proliferation. Regarding CD90 expression, more than 50% of the primary cells in Medium D and Medium M tested positive for this marker, whereas this population only accounted for about 25% of the primary cells grown in Medium DB. These CD90+cells were considered as contaminant cells in the breast carcinoma primary culture Araki et al., 2007Haack- Sorensen et al., 2008Nakamura et al., 2006. In sum, a marked contrast was observed with respect to the proportion of contaminant cells versus cells with the BCSC phenotype (CD44+CD24- and CD49f+) in primary cultures grown in Medium DB and Medium M.
Next, cellular ALDH expression was monitored to evaluate culture efficiency. The ALDH enzyme has important functions in the development of epithelial homeostasis, and deregulation of this class of enzymes has been implicated in multiple cancers Marchitti et al., 2008. The ALDEFLUOR assay is thought to be an almost universal marker of stem cell activity in both normal and cancer tissues Corti et al., 2006Hess et al.,2004, including normal and malignant breast epithelial stem cells Ginestier et al.,2007. In this study, the ALDH+cell population was approximately 5 times larger in Medium DB than in Medium M (9.35% ± 3.64% and 2.28% ± 0.88%, respectively). To sum up, Medium DB, significantly more than the other 2 media, specifically promotes the growth of the breast cancer stem cells that exist in malignant breast tumours. To support this conclusion, karyotype analysis revealed that nearly all cells in Medium DB exhibited an abnormal karyotype, while in Medium M as well as Medium D, primary cells contained both normal and slightly abnormal karyotypes. All samples doing karyotype derived from female breast cancer patients whose tumors were diagnosed as primary tumors, i.e. they had never undergone any previous treatment, including chemotherapy or radiotherapy. Consequently, the number of chromosomes of primary cancer cells was not so different than the normal chromosome number, known as 46 chromosomes. This is consistent with many studies of cancer cells primary culture, including Adeyinka et al., 2000Bardi et al., 1993Brothman et al., 1990Ferti et al.,2004Stamouli et al., 2004Teixeira et al., 1995.
Following karyotyping, primary cells from Medium M and Medium DB were used to induce tumours in mice. The results showed that primary cells grown in either Medium M or Medium DB, as well as other BCC lines such as MCF-7 and MDA-MD-231 successfully caused tumours in mice when injected at a cell density of 106 cells per mouse. Lower densities of primary cells or BCC lines failed to establish tumours in mice. Mouse models that were used in the experiments of examining the dose causing tumors were athymic nude mice, whose immune system is partially suppressed. Therefore, human cell transplantation, primary cells or BCC lines, induced immune response in mice, with the most powerful after 1 week. As a result, grafted cells including primary cells and BCC lines existed only 2 weeks. However, the results also showed the ability to establish tumors in mouse model with primary cells cultured in Medium DB and Medium M was as well as BCC lines. Tumour sections stained with HE confirmed that the tumours contained cancer cells with large nuclei; the tumours were established from primary cells cultured in Medium DB and Medium M.
Moreover, after long-term cultivation of approximately 6 months in Medium DB, breast cancer cells were observed to undergo the process of MET, i.e. altering their mesenchymal phenotype back to the epithelial phenotype. This process continued until epithelial cell colonies were formed, and eventually all cells in the culture flask transformed into the epithelial phenotype. At this point, cells proliferated more rapidly and stably, in a manner similar to that observed in typical BCC lines. Epithelial cancer cells acquire mesenchymal features that provide a mechanism for tumour cells to leave the primary tumour, resulting in the induction of single cell and/or collective cell migration Drasin et al., 2011Mani et al., 2008May et al., 2011. Overall, the carcinoma epithelial-mesenchymal transition (EMT) is defined by a loss of normal epithelial architecture, which renders the epithelial tumour cells phenotypically indistinguishable from fibroblasts. At the molecular level, this is characterized by a downregulation of epithelial markers and an up-regulation of mesenchymal markers, accompanied by an increase in cell migration and invasion Polyak and Weinberg,2009Scheel and Weinberg, 2011Thiery et al., 2009. However, the role of mesenchymal-epithelial transition (EMT) in cancer is complicated by the fact that in the appropriate microenvironment, mesenchymal-like cells likely undergo a reversion or MET, thereby permitting colonization Micalizzi et al., 2010Wells et al., 2008.
Conclusion
BCCs are essential biological tools for both research and therapy. Breast tumours always contain a combination of different kinds of cells, including breast cancer cells. This study successfully established a simple cell isolation procedure based on breast tumourexplant cultivation in Medium DB, which is a 1:1 mixture of DMEM/F12 supplemented with 10% FBS and M171 supplemented with 1X MEGS. Cells isolated using this procedure exhibited BCC properties such as expression of BCSC markers (CD44+CD24-), expression of ALDH, abnormal karyotype, and tumourigenic capability in mouse models. The findings of this study served to establish a method that proved highly useful for the isolation of BCCs from tumour biopsies as well as for the enrichment of BCSCs.
Abbreviations
ALDH: Aldehyde dehydrogenase, APC: Allophycocyanin, BCC: Breast cancer cell, BCSC: Breast cancer stem cell, BPE: Bovine pituitary extract, DEAB: Diethylaminobenzaldehyde, DMEM/F-12: Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12, EGF: Epithelial growth factor, EMT: Epithelialmesenchymal transition, FBS: Fetal Bovine Serum , FCM: Flow cytometry, FITC: Fluorescein Isothiocyanate, HE: Hematoxylineosin, HBC: Human breast cancer cell, HME: Human mammary epithelial cells, Medium D: DMEM/F12 supplemented with 10%FBS and 1% Antibiotics/antimycotic, Medium M: Medium 171 supplemented with MEGS and 1% Antibiotics/antimycotic, Medium DB Medium D and Medium M (1:1, v/v), MEGS: Mammary Epithelial Growth Supplement, MET: Mesenchymal-epithelial transition, NOD/SCID: Non-obese diabetic/severe combined immune deficiency.
References
-
A.
Adeyinka,
S.
Kytola,
F.
Mertens,
N.
Pandis,
C.
Larsson.
Spectral karyotyping and chromosome banding studies of primary breast carcinomas and their lymph node metastases. International journal of molecular medicine.
2000;
5
:
235-240
.
-
M.
Al-Hajj,
M.S.
Wicha,
A.
Benito-Hernandez,
S.J.
Morrison,
M.F.
Clarke.
Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America.
2003;
100
:
3983-3988
.
-
H.
Araki,
K.
Yoshinaga,
P.
Boccuni,
Y.
Zhao,
R.
Hoffman,
N.
Mahmud.
Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood.
2007;
109
:
3570-3578
.
-
V.
Band,
R.
Sager.
Distinctive traits of normal and tumorderived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proceedings of the National Academy of Sciences of the United States of America.
1989;
86
:
1249-1253
.
-
G.
Bardi,
B.
Johansson,
N.
Pandis,
N.
Mandahl,
E.
Bak-Jensen,
A.
Andren-Sandberg,
F.
Mitelman,
S.
Heim.
Karyotypic abnormalities in tumours of the pancreas. British journal of cancer.
1993;
67
:
11061112
.
-
J.
Bartek,
J.
Taylor-Papadimitriou,
N.
Miller,
R.
Millis.
Patterns of expression of keratin 19 as detected with monoclonal antibodies in human breast tissues and tumours. International journal of cancer Journal international du cancer.
1985;
36
:
299-306
.
-
S.
Bomken,
K.
Fiser,
O.
Heidenreich,
J.
Vormoor.
Understanding the cancer stem cell. British journal of cancer.
2010;
103
:
439-445
.
-
A.R.
Brothman,
D.M.
Peehl,
A.M.
Patel,
J.E.
McNeal.
Frequency and pattern of karyotypic abnormalities in human prostate cancer. Cancer research.
1990;
50
:
3795-3803
.
-
M.F.
Clarke.
A self-renewal assay for cancer stem cells. Cancer chemotherapy and pharmacology 56 Suppl.
2005;
1
:
64-68
.
-
S.
Corti,
F.
Locatelli,
D.
Papadimitriou,
C.
Donadoni,
S.
Salani,
R.
Del Bo,
S.
Strazzer,
N.
Bresolin,
G.P.
Comi.
Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem cells.
2006;
24
:
975-985
.
-
D.J.
Drasin,
T.P.
Robin,
H.L.
Ford.
Breast cancer epithelial-to-mesenchymal transition: examining the functional consequences of plasticity. Breast cancer research: BCR.
2011;
13
:
226
.
-
L.W.
Engel,
N.A.
Young.
Human breast carcinoma cells in continuous culture: a review. Cancer research.
1978;
38
:
4327-4339
.
-
S.P.
Ethier,
M.L.
Mahacek,
W.J.
Gullick,
T.S.
Frank,
B.L.
Weber.
Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer research.
1993;
53
:
627-635
.
-
S.P.
Ethier,
R.M.
Summerfelt,
K.C.
Cundiff,
B.B.
Asch.
The influence of growth factors on the proliferative potential of normal and primary breast cancer-derived human breast epithelial cells. Breast cancer research and treatment.
1991;
17
:
221-230
.
-
A.D.
Ferti,
M.J.
Stamouli,
A.D.
Panani,
S.A.
Raptis,
B.D.
Young.
Molecular cytogenetic analysis of breast cancer: a combined multicolor fluorescence in situ hybridization and G-banding study of uncultured tumor cells. Cancer genetics and cytogenetics.
2004;
149
:
28-37
.
-
H.
Ghebeh,
G.M.
Sleiman,
P.S.
Manogaran,
A.
Al-Mazrou,
E.
Barhoush,
F.H.
Al-Mohanna,
A.
Tulbah,
K.
Al-Faqeeh,
C.N.
Adra.
Profiling of normal and malignant breast tissue show CD44high/CD24low phenotype as a predominant stem/progenitor marker when used in combination with Ep-CAM/CD49f markers. BMC cancer.
2013;
13
:
289
.
-
J.P.
Gillet,
A.M.
Calcagno,
S.
Varma,
M.
Marino,
L.J.
Green,
M.I.
Vora,
C.
Patel,
J.N.
Orina,
T.A.
Eliseeva,
V.
Singal.
Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proceedings of the National Academy of Sciences of the United States of America.
2011;
108
:
1870818713
.
-
J.P.
Gillet,
S.
Varma,
M.M.
Gottesman.
The clinical relevance of cancer cell lines. Journal of the National Cancer Institute.
2013;
105
:
452-458
.
-
C.
Ginestier,
M.H.
Hur,
E.
Charafe-Jauffret,
F.
Monville,
J.
Dutcher,
M.
Brown,
J.
Jacquemier,
P.
Viens,
C.G.
Kleer,
S.
Liu.
ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell.
2007;
1
:
555-567
.
-
M.
Haack-Sorensen,
T.
Friis,
L.
Bindslev,
S.
Mortensen,
H.E.
Johnsen,
J.
Kastrup.
Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy. Scandinavian journal of clinical and laboratory investigation.
2008;
68
:
192-203
.
-
S.L.
Hammond,
R.G.
Ham,
M.R.
Stampfer.
Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proceedings of the National Academy of Sciences of the United States of America.
1984;
81
:
54355439
.
-
D.A.
Hess,
T.E.
Meyerrose,
L.
Wirthlin,
T.P.
Craft,
P.E.
Herrbrich,
M.H.
Creer,
J.A.
Nolta.
Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood.
2004;
104
:
1648-1655
.
-
P.J.
Keller,
A.F.
Lin,
L.M.
Arendt,
I.
Klebba,
A.D.
Jones,
J.A.
Rudnick,
T.A.
DiMeo,
H.
Gilmore,
D.M.
Jefferson,
R.A.
Graham.
Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast cancer research: BCR.
2010;
12
:
R87
.
-
M.
Lacroix,
G.
Leclercq.
Relevance of breast cancer cell lines as models for breast tumours: an update. Breast cancer research and treatment.
2004;
83
:
249-289
.
-
S.A.
Mani,
W.
Guo,
M.J.
Liao,
E.N.
Eaton,
A.
Ayyanan,
A.Y.
Zhou,
M.
Brooks,
F.
Reinhard,
C.C.
Zhang,
M.
Shipitsin.
The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell.
2008;
133
:
704-715
.
-
F.
Mannello.
Understanding breast cancer stem cell heterogeneity: time to move on to a new research paradigm. BMC medicine.
2013;
11
:
169
.
-
S.A.
Marchitti,
C.
Brocker,
D.
Stagos,
V.
Vasiliou.
Non- P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert opinion on drug metabolism & toxicology.
2008;
4
:
697-720
.
-
A.
Marusyk,
K.
Polyak.
Tumor heterogeneity: causes and consequences. Biochimica et biophysica acta.
2010;
1805
:
105-117
.
-
C.D.
May,
N.
Sphyris,
K.W.
Evans,
S.J.
Werden,
W.
Guo,
S.A.
Mani.
Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast cancer research: BCR.
2011;
13
:
202
.
-
M.J.
Meyer,
J.M.
Fleming,
A.F.
Lin,
S.A.
Hussnain,
E.
Ginsburg,
B.K.
Vonderhaar.
CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer research.
2010;
70
:
4624-4633
.
-
D.S.
Micalizzi,
S.M.
Farabaugh,
H.L.
Ford.
Epithelialmesenchymal transition in cancer: parallels between normal development and tumor progression. Journal of mammary gland biology and neoplasia.
2010;
15
:
117-134
.
-
Y.
Nakamura,
Y.
Muguruma,
T.
Yahata,
H.
Miyatake,
D.
Sakai,
J.
Mochida,
T.
Hotta,
K.
Ando.
Expression of CD90 on keratinocyte stem/progenitor cells. The British journal of dermatology.
2006;
154
:
1062-1070
.
-
R.M.
Neve,
K.
Chin,
J.
Fridlyand,
J.
Yeh,
F.L.
Baehner,
T.
Fevr,
L.
Clark,
N.
Bayani,
J.P.
Coppe,
F.
Tong.
A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer cell.
2006;
10
:
515-527
.
-
O.W.
Petersen,
B.
van Deurs.
Preservation of defined phenotypic traits in short-term cultured human breast carcinoma derived epithelial cells. Cancer research.
1987;
47
:
856-866
.
-
K.
Polyak,
R.A.
Weinberg.
Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews Cancer.
2009;
9
:
265-273
.
-
C.
Scheel,
R.A.
Weinberg.
Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells?. International journal of cancer Journal international du cancer.
2011;
129
:
2310-2314
.
-
H.S.
Smith,
S.
Lan,
R.
Ceriani,
A.J.
Hackett,
M.R.
Stampfer.
Clonal proliferation of cultured nonmalignant and malignant human breast epithelia. Cancer research.
1981;
41
:
4637-4643
.
-
H.S.
Smith,
S.R.
Wolman,
S.H.
Dairkee,
M.C.
Hancock,
M.
Lippman,
A.
Leff,
A.J.
Hackett.
Immortalization in culture: occurrence at a late stage in the progression of breast cancer. Journal of the National Cancer Institute.
1987;
78
:
611-615
.
-
H.D.
Soule,
J.
Vazguez,
A.
Long,
S.
Albert,
M.
Brennan.
A human cell line from a pleural effusion derived from a breast carcinoma. Journal of the National Cancer Institute.
1973;
51
:
1409-1416
.
-
M.I.
Stamouli,
A.D.
Panani,
A.D.
Ferti,
C.
Petraki,
R.T.
Oliver,
S.A.
Raptis,
B.D.
Young.
Detection of genetic alterations in primary bladder carcinoma with dual-color and multiplex fluorescence in situ hybridization. Cancer genetics and cytogenetics.
2004;
149
:
107-113
.
-
M.R.
Stampfer.
Cholera toxin stimulation of human mammary epithelial cells in culture. In vitro.
1982;
18
:
531-537
.
-
M.R.
Stampfer,
J.C.
Bartholomew,
H.S.
Smith,
J.C.
Bartley.
Metabolism of benzo[a]pyrene by human mammary epithelial cells: toxicity and DNA adduct formation. Proceedings of the National Academy of Sciences of the United States of America.
1981a;
78
:
6251-6255
.
-
M.R.
Stampfer,
J.C.
Bartley.
Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proceedings of the National Academy of Sciences of the United States of America.
1985;
82
:
2394-2398
.
-
M.R.
Stampfer,
I.
Vlodavsky,
H.S.
Smith,
R.
Ford,
F.F.
Becker,
J.
Riggs.
Fibronectin production by human mammary cells. Journal of the National Cancer Institute.
1981b;
67
:
253-261
.
-
M.R.
Stampfer,
P.
Yaswen,
M.
Alhadeff,
J.
Hosoda.
TGF beta induction of extracellular matrix associated proteins in normal and transformed human mammary epithelial cells in culture is independent of growth effects. Journal of cellular physiology.
1993;
155
:
210-221
.
-
S.Y.
Sung,
C.L.
Hsieh,
D.
Wu,
L.W.
Chung,
P.A.
Johnstone.
Tumor microenvironment promotes cancer progression, metastasis, and therapeutic resistance. Current problems in cancer.
2007;
31
:
36100
.
-
J.
Taylor-Papadimitriou,
M.
Stampfer,
J.
Bartek,
A.
Lewis,
M.
Boshell,
E.B.
Lane,
I.M.
Leigh.
Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. Journal of cell science 94(Pt.
1989;
3)
:
403-413
.
-
M.R.
Teixeira,
N.
Pandis,
G.
Bardi,
J.A.
Andersen,
F.
Mitelman,
S.
Heim.
Clonal heterogeneity in breast cancer: karyotypic comparisons of multiple intra- and extra-tumorous samples from 3 patients. International journal of cancer Journal international du cancer.
1995;
63
:
6368
.
-
J.P.
Thiery,
H.
Acloque,
R.Y.
Huang,
M.A.
Nieto.
Epithelial-mesenchymal transitions in development and disease. Cell.
2009;
139
:
871-890
.
-
C.E.
Weber,
P.C.
Kuo.
The tumor microenvironment. Surgical oncology.
2012;
21
:
172-177
.
-
A.
Wells,
C.
Yates,
C.R.
Shepard.
E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clinical & experimental metastasis.
2008;
25
:
621-628
.
-
S.R.
Wolman,
H.S.
Smith,
M.
Stampfer,
A.J.
Hackett.
Growth of diploid cells from breast cancers. Cancer genetics and cytogenetics.
1985;
16
:
49-64
.
-
H.
Yu,
J.K.
Mouw,
V.M.
Weaver.
Forcing form and function: biomechanical regulation of tumor evolution. Trends in cell biology.
2011;
21
:
47-56
.
-
K.R.
Yu,
S.R.
Yang,
J.W.
Jung,
H.
Kim,
K.
Ko,
D.W.
Han,
S.B.
Park,
S.W.
Choi,
S.K.
Kang,
H.
Scholer.
CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem cells.
2012;
30
:
876-887
.
Comments

Downloads
Article Details
Volume & Issue : Vol 2 No 02 (2015)
Page No.: 207-219
Published on: 2015-02-22
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 9340 times
- Download PDF downloaded - 1911 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress