Abstract
Background: Urinary bladder cancer (UBC) is the ninth most frequently diagnosed cancer worldwide. Early diagnosis and treatment can improve survival of patients. In order to improve the diagnostic accuracy of non-invasive urinary bladder cancer, a large number of tumor markers have been identified and strictly assessed. Some of the best candidates as predictive markers in oncologic diseases belong to the family of matrix metalloproteinases (MMPs). The main focus of investigation in this study was on MMP-1, MMP-2, MMP-3, MMP-8, MMP-9 and tissue inhibitor of metalloproteinase 1 (TIMP-1) as plasma biomarkers in patients with urinary bladder cancer (depending on tumor stage).
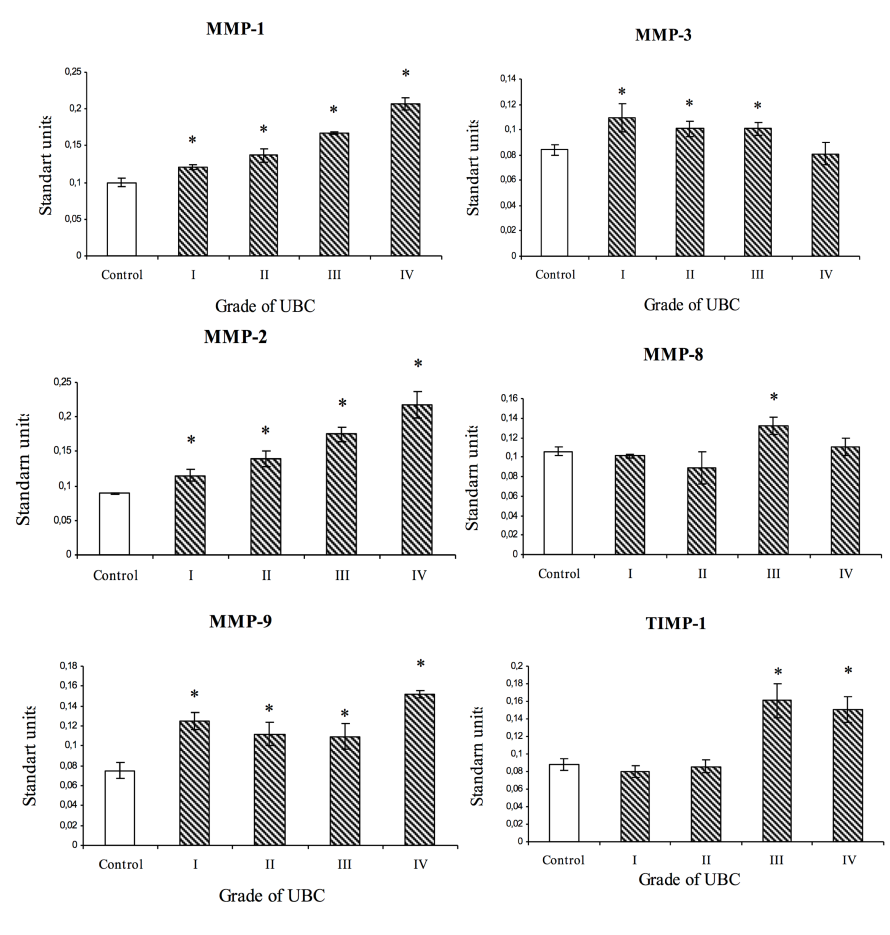
Methods: Plasma levels of MMP-9 were significantly higher in all patients with UBC compared to control subjects. The plasma level of MMP-8 in Stage III UBC patients was 1.2 times higher than in control group. The plasma level of MMP-3 was higher in patients with bladder cancer of Stage I, II or III (compared to control subjects). Moreover, high plasma levels of TIMP-1 were observed in patients with UBC stages III and IV.
Results: Overall, the measurements of circulating blood levels of MMP-1 and MMP-2 are progressively dissimilar among the various groups (UBC versus control subjects). Thus, changes in MMP levels may be used for monitoring and/or predicting progression of UBC.
Introduction
Urinary bladder cancer (UBC) is a common disease worldwide with a high mortality rate Ploeg et al., 2009. UBC is the ninth most frequently diagnosed cancer worldwide, with the highest incidence rates observed in developed countries. About 75% of bladder cancer cases occur in men Murta-Nascimento et al., 2007. To date, the research of bladder cancer tumor markers has rapidly evolved. Indeed, a vast number of tumor markers have been identified and rigorously evaluated in the attempt to improve noninvasive diagnostic accuracy in bladder cancer.
Of the tumor markers for bladder cancer, matrix metalloproteinases (ММРs) are among the best candidates. To date, there has been a large number of published scientific studies dedicated to investigating intracellular markers. ММРs are capable of destroying and increasing the permeability of the cytomatrix by means of splitting its components (e.g. collagen, proteoglycans, elastin, laminin, and fibronectin). Tumor cells release ММРs which split these cytomatrix components and induce invasion/metastasis Shirodkar et al., 2009Rodriguez Faba et al., 2012. MMPs are also involved in the earlier stages of tumor progression, such as carcinomatous degeneration, angiogenesis, and tumor growth at primary and metastatic sites Hua et al., 2011. The search for diagnostic and predictive markers for better identification of patients with poor chance of survival would be useful for customization of pre- and post-surgery therapy.
The aim of the present study was to assess the clinical usefulness of plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinase 1 (TIMP-1) in the diagnosis and prognosis of UBC.
Materials - Methods
This study enrolled 29 bladder cancer patients (27 males, 2 females), with age ranging from 52-76 years. All patients underwent standard preoperative examinations including blood and urine analysis, blood chemistry, cancer immunograms, сomputed tomography (CT) of chest, abdomen and pelvis (with contrast medium to assess the spread of the cancer), and tumor biopsy with pathological evaluation. Bladder cancer stages were classified in accordance with the TNM clinical classification (7th edition, 2009). The study subjects were classified as follows:
Stage I (9 patients)
Stage II (7 patients)
Stage III (7 patients)
Stage IV (8 patients)
The control group consisted of 30 healthy individuals (8 females and 22 males) of ages from 30-35 years.
Plasma levels of MMP-1, MMP-2, MMP-3, MMP-8, MMP-9 and TIMP-1 were determined by ELISA before the definitive treatment and correlated afterwards with clinical parameters of the patient. The sample volume of 100 ml was incubated in 96-well plates overnight at 4ºC. Primary polyclonal antibodies (Santa Cruz Biotechnology, USA) were used, as were secondary antibodies conjugated with horseradish peroxidase (Bio-Rad, USA) and phenylenediamine substrate hydrogen peroxide (Sigma-Aldrich, Louis St, MO). The measurements of the ELISA assay were carried out at a wavelength of 492 nm.
Statistical analysis of the results was performed using methods of variation statistics; correlation analysis was done using Excel program. To determine the reliability of the differences between two samples we used the Student’s t-test. The results were considered statistically significant if P < 0.05.
Results
Plasma levels of MMP-9 were significantly higher (by 1.5-2.0 times) in all patients with UBC compared to controls ( Figure 1 ). The plasma level of MMP-8 in Stage III UBC patients was 1.2 times greater than for the control group. In fact, the plasma levels of MMP-1 were significantly higher by 1.21-, 1.37-, 1.67- and 2.07-fold for Stage I, II, III and IV patients, respectively. Moreover, high plasma levels of MMP-2 were seen in patients with bladder cancer Stage I, II, III and IV, versus controls (by 1.3, 1.5, 1.9 and 2.4-fold, respectively). The levels of MMP-1 and MMP-2 were correlated with increase in cancer stage. Furthermore, the plasma level of MMP-3 in patients with bladder cancer Stage I, II and III were higher compared to controls (by 1.3-, 1.2- and 1.2-fold, respectively). High plasma levels of TIMP-1 were also observed in patients with Stage III and IV.
Discussion
For invasion and metastasis, tumor cells have some key properties for breaking through biological barriers; one is the ability to penetrate into surrounding tissues (including blood vessels or lymph tubes) and another is the ability to survive after entering into vessels and forming a new focal site for tumor growth. The integral part of many physiological processes, including tissue healing and regeneration, is extracellular matrix degradation. This process is also necessary for tumor invasion as the basic structural proteins of the intracellular matrix (ECM) are the major barrier.
In the degradation of the extracellular matrix, there is participation of several families of peptidases, the most important belonging to the family of MMPs Stivarou et al., 2015Martin et al., 2013Keleg et al., 2003. The role of ММРs in tumor proliferation and metastasis was initially defined by Liotta et al. in early 1980s as a result of identifying the participation of hydrolysis of IV type collagen in the process of invasion and metastasis of melanoma Liotta et al., 1980. The MMP multigene family is composed of over 20 calcium-dependent zinc-containing endopeptidases. The activity of ММРs are regulated by two types of inhibitors: TIMP-1 and α2-macroglobulin Pittayapruek et al., 2016. Moreover, collagenase-1 is the major secreted neutral proteinase capable of initiating degradation of natural collagen of type I, II, III and V. It plays a leading role in degradation of ECM collagen in various physiological and pathological processes.
Expression of ММР-1 in vitro is observed in active remodeling areas. ММР-1 is expressed by several types of cells, including fibroblasts, keratinocytes, cartilage cells, monocytes, hepatocytes and various tumor cells Sunamia et al., 2000. For breast cancer, it has been proposed that MMP-1 is a potential biomarker Decock et al., 2008. Damian Pollard et al. have reported a great increase in serum levels of MMP-1 in patients with lung cancer Pollard et al., 2013. High levels of MMP-1 expression in patients with colorectal cancer was also associated with a poor prognosis Sunamia et al., 2000. In the present study we demonstrated that the plasma levels of MMP-1 in urinary bladder cancer patients increased according to the tumor stage and could be a strong prognostic marker for UBC.
MMP-2 and MMP-9 are members of gelatinases. ММP-2 and ММP-9 play major roles in the neo-angiogenesis process and initiate angiogenesis in early stages of tumor vascularization Page-McCaw et al., 2007. Hrabec et al. have reported that the levels of plasma and serum MMP-9 in patients with lung cancer are significantly higher than those in the control group Hrabec et al., 2001. High levels of MMP-9 have been found in blood samples from patients with breast, colorectal and gastric cancers, and melanoma Kostova et al., 2012Holanda et al., 2017Nikkola et al., 2005Mroczko et al., 2009.
In our study cancer patients had significantly higher MMP-9 levels compared to control patients, regardless of tumor stage. It is possible that this factor might be involved and dominate in early tumor progression stages. In our study we demonstrated that the plasma levels of MMP-2 in bladder cancer patients increased according to tumor stage; thus, it may serve as a useful and representative prognostic marker for UBC. Several studies have assessed the predictive value of MMP-2 but have arrived at opposite results. Several authors have reported the increase of plasma/serum levels of MMP-2 in patients with colorectal cancer, melanoma, and breast and gastric cancers Kostova et al. ,2012Holanda et al., 2017, Roopali et al., 2009. On the other hand, Kuvaja et al. reported that low serum levels of circulating MMP-2 and TIMP-2 are associated with a poor prognosis in bladder cancer patients Kuvaja et al., 2008. Also, Ylisirniö et al. have reported reduced serum levels of TIMP-2 and MMP-2/TIMP-2 complex in cancer patients compared to healthy controls Ylisirniö et al., 2000.
MMP-3, also known as stromelysin-1, catalyzes the degradation of many components of connective tissue, including proteoglycans, link protein, collagen of II, IV, IX and XI types, laminin, and fibronectin. It is also believed that this enzyme participates in wound repair, atherosclerosis progression and tumor initiation. MMP-3 can activate other MMPs, such as MMP-1, MMP-7 and MMP-9, and plays a key role in tissue remodeling Rose et al., 2016. Studies of cell cultures or in vivo models have demonstrated the participation of ММР-3 in the progression of pulmonary cancer, breast cancer, and pancreatic cancer. The correlation between expression of ММР-3 in tumors and disease progression or overall survival of patients is much less known Mehner et al., 2015, Huang et al., 2016. Our study shows increased circulating levels of MMP-3 in patients with Stage I, II and III bladder cancer.
ММР-8 is synthesized by polymorphonuclear leukocytes, accumulates in secretory granules, and is released in response to certain stimuli of a key enzyme (which starts destruction of the extracellular matrix, especially in the case of pathological inflammatory conditions like rheumatoid arthritis and osteoarthritis). Several studies have revealed the suppressive impact of ММР-8 on metastasis Klein et al., 2011. Moreover, they have demonstrated that MMP-8 expression is high in neck squamous and head cell carcinoma Decock et al., 2011. In patients with ovarian cancer, it has been shown that MMP-8 expression level correlates with poor prognosis and tumor stage Al-Alem et al., 2015. The levels of MMP-8 are increased in patients with Stage III bladder cancer.
There are data showing that TIMP-1 plays an important individual role in growth regulation/differentiation of tumor cells and normal cells. As well, it has anti-angiogenic properties Hua et al., 2011. The increased level of circulating TIMP-1 has been observed in many malignant tumors and related to unfavorable prognosis (as TIMP-1 is a metastasis inhibitor) Gong et al., 2014. In their study, Rauvala et al. demonstrated that the level of TIMP-1 in blood/serum of pre-surgery patients correlates with the stage of ovarian cancer Rauvala et al., 2005. In patients with head and neck squamous cell cancer. the level of TIMP-1 in tissues and serum are associated with unfavorable prognosis (Ruokolainen et al., 2005). Our present study demonstrates that levels of TIMP-1 are significantly elevated in plasma (by 1.7- and 1.8- fold) in patients with stage IV and III UBC, respectively, compared to controls. This significant TIMP-1 increase may have a protective function in processes of invasion by inhibiting extracellular matrix degradation.
Conclusion
In this study we investigated the potential prognostic value of MMP-9, MMP-8, MMP-3, MMP-2, MMP-1 and TIMP-1 in bladder cancer progression. The plasma levels of circulating MMP-1 and MMP-2 were progressively different in bladder cancer patients of different stages, suggesting that changes in MMP level can be used for monitoring or predicting the progression of UBC. The levels of MMP-1 and MMP-2 were increased according to the tumor stage and, thus, could be potential markers of UBC progression in patients. Unfortunately, in spite of numerous confirmations that TIMP-1 is a strong prognostic marker, in patients with UBC the level of this inhibitor was increased only in those with Stage III and IV. These markers should be further studied for their potential usefulness for the examination and monitoring of patients with UBC.
Author Contribution
All authors were equally contributed to the study design, bioinformatics analysis, drafting of the manuscript and approved the manuscript for publication.
References
-
L.
Al-Alem,
T. E. Jr.
Curry.
Ovarian cancer: Involvement of the matrix metalloproteinases. Reproduction (Cambridge.
2015;
England)
:
150(2)
.
View Article PubMed Google Scholar -
J.
Decock,
W.
Hendrickx,
U.
Vanleeuw,
V.
Van Belle,
S.
Van Huffel,
M.R.
Christiaens,
S. Paridaens
Ye.
Plasma MMP1 and MMP8 expression in breast cancer: Protective role of MMP8 against lymph node metastasis. BMC Cancer.
2008;
8(1)
:
77
.
View Article PubMed Google Scholar -
J.
Decock,
S.
Thirkettle,
L.
Wagstaff,
D. R.
Edwards.
Matrix metalloproteinases: Protective roles in cancer. Journal of Cellular and Molecular Medicine.
2011;
15(6)
:
1254-1265
.
View Article PubMed Google Scholar -
Y.
Gong,
U. D.
Chippada-Venkata,
W. K.
Oh.
Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel).
2014;
6(3)
:
1298-1327
.
View Article PubMed Google Scholar -
A. O.
Holanda,
A. R.
Oliveira,
K. J.
Cruz,
J. S.
Severo,
J. B.
Morais,
B. B.
Silva,
D. D.
Marreiro.
Zinc and metalloproteinases 2 and 9: What is their relation with breast cancer?. Revista da Associação Médica Brasileira.
2017;
63(1)
:
78-84
.
-
E.
Hrabec,
M.
Strek,
D.
Nowak,
Z.
Hrabec.
Elevated level of circulating matrix metalloproteinase-9 in patients with lung cancer. Respiratory Medicine.
2001;
95(1)
:
1-4
.
View Article PubMed Google Scholar -
H.
Hua,
M.
Li,
T.
Luo,
Y.
Yin,
Y.
Jiang.
Matrix metalloproteinases in tumorigenesis: An evolving paradigm. Cellular and Molecular Life Sciences.
2011;
68(23)
:
3853-3868
.
View Article PubMed Google Scholar -
J.-F.
Huang,
W. X.
Du,
J. J.
Chen.
Elevated expression of matrix metalloproteinase-3 in human osteosarcoma and its association with tumor metastasis. Journal of the Balkan Union of Oncology.
2016;
21(1)
:
235-243
.
PubMed Google Scholar -
S.
Keleg,
P.
Büchler,
R.
Ludwig,
M. W.
Büchler,
H.
Friess.
Invasion and metastasis in pancreatic cancer. Molecular Cancer.
2003;
2(1)
:
14
.
View Article PubMed Google Scholar -
T.
Klein,
R.
Bischoff.
Physiology and pathophysiology of matrix metalloproteases. Amino Acids.
2011;
41(2)
:
271-290
.
View Article PubMed Google Scholar -
E.
Kostova,
M.
Slaninka Miceska,
N.
Labacevski,
K.
Jakjovski,
J.
Trojacanec,
E.
Atanasovska,
V.
Janevski,
R.
Selmani,
G.
Petrushevska,
V.
Janevska.
. Journal of Health Sciences (Sarajevo).
2012;
2(3)
:
169-175
.
-
L. A.
Liotta,
K.
Tryggvason,
S.
Garbisa,
I.
Hart,
C. M.
Foltz,
S.
Shafie.
Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature.
1980;
284(5751)
:
67-68
.
View Article PubMed Google Scholar -
T.A.
Martin,
L.
Ye,
A.J.
Sanders,
J.
Lane,
W.G.
Jiang.
Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In R. Jandial (Ed.), Metastatic Cancer: Clinical and Biological Perspectives. Austin, Texas: Landes Bioscience.
2013
.
-
C.
Mehner,
E.
Miller,
A.
Nassar,
W. R.
Bamlet,
E. S.
Radisky,
D. C.
Radisky.
Tumor cell expression of MMP3 as a prognostic factor for poor survival in pancreatic, pulmonary, and mammary carcinoma. Genes & Cancer.
2015;
6(11-12)
:
480-489
.
PubMed Google Scholar -
B.
Mroczko,
M.
Groblewska,
M.
Łukaszewicz-Zajac,
R.
Bandurski,
B.
Ke˛dra,
M.
Szmitkowski.
Pre-treatment serum and plasma levels of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) in gastric cancer patients. Clinical Chemistry and Laboratory Medicine.
2009;
47(9)
:
1133-1139
.
View Article PubMed Google Scholar -
C.
Murta-Nascimento,
B. J.
Schmitz-Dräger,
M. P.
Zeegers,
G.
Steineck,
M.
Kogevinas,
F. X.
Real,
N.
Malats.
Epidemiology of urinary bladder cancer: From tumor development to patient’s death. World Journal of Urology.
2007;
25(3)
:
285-295
.
View Article PubMed Google Scholar -
J.
Nikkola,
P.
Vihinen,
M. S.
Vuoristo,
P.
Kellokumpu-Lehtinen,
V. M.
Kähäri,
S.
Pyrhönen.
High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clinical Cancer Research.
2005;
11(14)
:
5158-5166
.
View Article PubMed Google Scholar -
A.
Page-McCaw,
A. J.
Ewald,
Z.
Werb.
Matrix metalloproteinases and the regulation of tissue remodelling. Nature Reviews. Molecular Cell Biology.
2007;
8(3)
:
221-233
.
-
P.
Pittayapruek,
J.
Meephansan,
O.
Prapapan,
M.
Komine,
M.
Ohtsuki.
Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. International Journal of Molecular Sciences.
2016;
17(6)
:
868
.
View Article PubMed Google Scholar -
M.
Ploeg,
K. K.
Aben,
L. A.
Kiemeney.
The present and future burden of urinary bladder cancer in the world. World Journal of Urology.
2009;
27(3)
:
289-293
.
View Article PubMed Google Scholar -
D
Pollard.
Lung cancer biomarker discovery using proteomic techniques. Lung cancer biomarker discovery using proteomic techniques. PhD thesis, Dublin City University./ Damian Pollard, B.Sc. Hons Lung Cancer Biomarker Discovery using Proteomic Techniques.
2013
.
-
M.
Rauvala,
U.
Puistola,
T.
Turpeenniemi-Hujanen.
Gelatinases and their tissue inhibitors in ovarian tumors; TIMP-1 is a predictive as well as a prognostic factor. Gynecologic Oncology.
2005;
99(3)
:
656-663
.
View Article PubMed Google Scholar -
O
Rodriguez Faba,
J
Palou-Redorta,
JM
Fernández-Gómez,
F
Algaba,
N
Eiró,
H
Villavicencio,
FJ
Vizoso.
Matrix metalloproteinases and bladder cancer: what is new?. [online] ISRN Uro.
2012
.
PubMed Google Scholar -
BJ
Rose,
DL
Kooyman.
A tale of two joints: the role of matrix metalloproteases in cartilage biology. [online] Dis Markers.
2016
.
PubMed Google Scholar -
R.
Roy,
J.
Yang,
M. A.
Moses.
Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. Journal of Clinical Oncology.
2009;
27(31)
:
5287-5297
.
View Article PubMed Google Scholar -
H.
Ruokolainen,
P.
Pääkkö,
T.
Turpeenniemi-Hujanen.
Tissue inhibitor of matrix metalloproteinase-1 is prognostic in head and neck squamous cell carcinoma: Comparison of the circulating and tissue immunoreactive protein. Clinical Cancer Research.
2005;
11(9)
:
3257-3264
.
View Article PubMed Google Scholar -
S. P.
Shirodkar,
V. B.
Lokeshwar.
Potential new urinary markers in the early detection of bladder cancer. Current Opinion in Urology.
2009;
19(5)
:
488-493
.
View Article PubMed Google Scholar -
E.
Sunamia,
N.
Tsuno,
T.
Osada,
S.
Saito,
J.
Kitayama,
S.
Tomozawa,
T.
Tsuruo,
Y.
Shibata,
T.
Muto,
H.
Nagawa.
MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. The Oncologist.
2000;
5(2)
:
108-114
.
View Article PubMed Google Scholar -
T.
Stivarou,
E.
Patsavoudi.
Extracellular molecules involved in cancer cell invasion. Cancers (Basel).
2015;
7(1)
:
238-265
.
View Article PubMed Google Scholar -
K.
Vasala,
P.
Kuvaja,
T.
Turpeenniemi-Hujanen.
Low circulating levels of ProMMP-2 are associated with adverse prognosis in bladder cancer. Tumour Biology.
2008;
29(5)
:
279-286
.
View Article PubMed Google Scholar -
S.
Ylisirniö,
M.
Höyhtyä,
T.
Turpeenniemi-Hujanen.
Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer—TIMP-1 as a prognostic marker. Anticancer Research.
2000;
20(2B)
:
1311-1316
.
PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 1 (2018)
Page No.: 1931-1940
Published on: 2018-01-23
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6095 times
- Download PDF downloaded - 2015 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress