Abstract
Oral mucositis (OM) is one of the most common side effects after hematopoietic stem cell transplantation (HSCT) and palifermin is used for prophylactic use to prevent OM. We conducted a meta-analysis study that evaluates the efficacy of palifermin on OM after HSCT in hematologic malignancy patients. Databases of PubMed/Medline, Web of Science and Cochrane Library for English-language publications were searched for finding the relevant studies. The RevMan 5.3 software with random-effects models (odds ratio (ORs) and 95% confidence intervals (CIs)) was used for to estimate of the efficacy of palifermin in palifermin group compared with control group. Begg's and Egger's tests were used for assessment of bias between the studies. Ten studies were included in the meta-analysis study. The results of the meta-analyses showed that there were significant differences in OM (grade 1-4) [odds ratio (OR) = 0.17; 95%CI= 0.10,0.29; p <0.00001], OM (grade 2-4) [OR= 0.11; 95%CI= 0.05,0.24; p <0.00001], OM (grade 3-4) [OR= 0.22; 95%CI= 0.15,0.33; p <0.00001], after auto-HSCT for OM (grade 1-4) [OR= 0.13; 95%CI= 0.04, 0.35; p <0.0001], OM (grade 2-4) [OR= 0.03; 95%CI= 0.00, 0.21; p =0.0006] or OM (grade 3-4) [OR= 0.25; 95%CI= 0.13, 0.48; p <0.0001], after allo-HSCT for OM (grade 1-4) [OR= 0.23; 95%CI= 0.11, 0.51; p =0.0002], OM (grade 2-4) [OR= 0.14; 95%CI= 0.03, 0.74; p =0.012] or OM (grade 3-4) [OR= 0.19; 95%CI= 0.08, 0.46; p =0.0002] and fever [OR=0.51; 95%CI=0.29, 0.87; p = 0.01], but there were no significant differences in acute graft versus host disease (aGVHD) grades, infection and blood stream infection between two groups. The meta-analysis showed that palifermin was associated with reductions in the incidence and severity of OM and also was effective and safe on OM after allo- or auto-HSCT, but did not seem to effect on the incidence and severity of aGVHD.
Introduction
Hematopoietic stem cell transplantation (HSCT) with high-dose chemo-radiotherapy is frequently used in the treatment of patients with hematologic malignancies. During the conditioning regimen for HSCT (which includes total body irradiation or high dose chemotherapy), and immediately after the transplant, patients may present a variety of symptoms Lauritano et al., 2014. Oral mucositis (OM) as one of symptoms of HSCT, is a common and important adverse effect Filicko et al., 2003Jilani et al., 2014Radtke and Kolesar, 2005 and OM creates in approximately 70–80 % of patients receiving radiation-based conditioning regimens Epstein et al., 2012. The European Group for Blood and Marrow Transplantation (EBMT) Registry reported that there were annually around 23500 transplants including 38% of allogeneic SCT (allo-SCT) and 62% of autologous SCT (auto-SCT) Hołowiecki, 2008. Allo-SCT is a form of immunotherapy and as one of HSCT types has increased survival of patients with relapsed leukemia and high-risk leukemia in remission that graft versus host disease (GVHD) was one of the most important complaints involving vital organs and infections Kolb, 2017. Auto-SCT that is another HSCT type has become a well-established treatment for a variety of cancers Vitale et al., 2014. Severe mucositis may create pain requiring opioid analgesics, a higher risk of infection, total parenteral nutrition, increased hospital charges, and decreased quality of life in the patients Horsley et al., 2007. The incidence of severe OM in patients receiving high-dose myeloablative therapy with HSCT is 70-80% Woo et al., 1993. This incidence in pediatric patients is higher than adults Sonis and Clark, 1991. Palifermin as a recombinant human keratinocyte growth factor was approved in the European Union in October 2005 for prophylactic purpose to prevent severe OM in patients receiving high-dose therapy and auto-HSCT Jilani et al., 2014Czyzewski et al., 2014Kobbe et al., 2010. In patients with hematologic cancer, a palifermin dosage of 60 µg/kg/day (total six doses, three doses before the preparative regimen and three doses after the stem-cell infusion), significantly decreases the incidence and duration of severe OM that this setting has been confirmed by the Food and Drug Administration (FDA) Spielberger et al., 2004Stiff et al., 2006. The aim of this meta-analysis study was to evaluate the efficacy of palifermin on the incidence and severity of OM and acute GVHD after HSCT in hematologic malignancy patients in case-control or matched-control studies.
Materials and Methods
Search strategies
A comprehensive search was done with search terms included with “palifermin” and “hematopoietic stem cell transplantation or HSCT or stem-cell transplant or stem cell transplant or hematopoietic cell transplantation or HCT or hematopoietic stem cell transplant or stem cell transplantation or stem-cell transplantation or hematopoietic stem-cell transplantation” and “oral mucositis or mucositis” in databases of PubMed/Medline, Web of Science and Cochrane Library for English-language publications.
Study selection
Three authors revised selection of the studies. The first author (M.S) searched the studies and then the second author (M.R) blinded to the first reviewer. If there was any disagreement between the two authors, the third author (H.R.M) resolved the problem. All the articles of this study were examined for evaluation of the efficacy of palifermin on OM and/or aGVHD after HSCT in palifermin group compared with control group. The studies of this meta-analysis had to include the following inclusion criteria: a) case-control and control-matched studies (cohort, retrospective and non-randomized trial studies); b) human studies; c) the comparison of aGVHD and OM based on the World Health Organization (WHO) in two groups after HSCT; d) the studies reporting OM and/ or aGVHD in hematologic malignancies patients. Exclusion criteria: a) duplication of previous publications; b) the review and case series studies; c) randomized trial studies; d) the studies reporting OM and/or aGVHD in solid tumor patients.
Data Extraction
We collected the name of author, the year of publication, country, the number of patients in palifermin group, the number of patients in control group, age (range) of both groups, the percentage of the male in each group, the dose of palifermin and the type of HSCT for each study that met our criteria. The aGVHD and OM were graded according to the World Health Organization (WHO).
Statistical analyses
The data analysis (a random-effect model) was done by Review Manager 5.3 (RevMan 5.3, The Cochrane Collaboration, Oxford, United Kingdom) using odds ratio (OR) and 95% confidence intervals (CIs). The heterogeneity between estimations was calculated by the Q and I2 statistics that for the Q statistic, heterogeneity was considered for p < 0.1. The I2 statistic yields results ranging from 0 to 100% (0–25%, 25–50%, 50–75% and 75–100% showed no heterogeneity, moderate heterogeneity, large heterogeneity and extreme heterogeneity, respectively Egger et al., 1997. We graphically evaluated publication bias using funnel plots and quantitatively evaluated bias using Begg's and Egger's tests. P < 0.05 (two-sided) was considered indicative of statistically significant publication bias.
Results
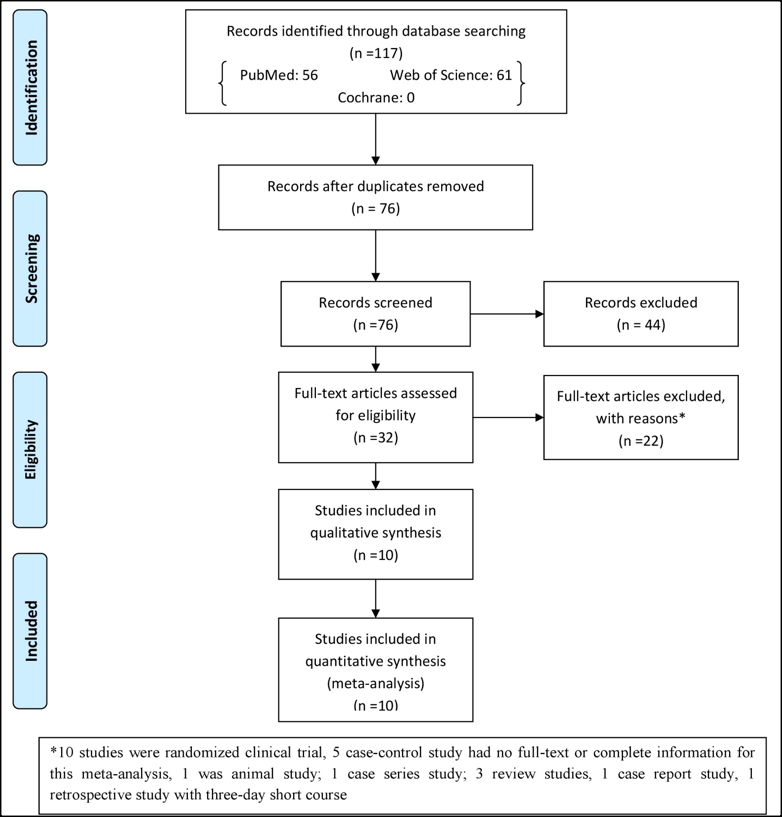
The search in the databases resulted in 117 studies that out of these studies, 32 studies were eligible for assessment. Out of 32 studies, 22 studies were excluded after searching and reading of full-text that the reasons for excluding have been written in Figure 1 . Therefore, 10 studies were included in meta-analysis study. One study was commentary study that had complete information for meta-analysis.
Studies characteristics
Table 1 shows the characteristics of 10 studies included in meta-analysis study. The studies were reported during 2007 to 2015. Three studies were reported in Poland, one in Netherlands, one in Australia, one in Austria, one in Italy, two in the USA, one in Greece, seven were case-control studies and three were matched-control studies. All studies were designed for hematologic malignancy patients that dose of palifermin for each study was 60μg/kg/day. This meta-analysis included 301 patients in control group and 364 patients in palifermin group. The age (range) and the percentage of the male for each group have been shown in Table 1 . Four studies were done undergoing auto-HSCT patients, four studies undergoing allo-HSCT patients, one study didn’t report the type of HSCT and one study reported both.
Meta–analysis: The incidence of OM and aGVHD
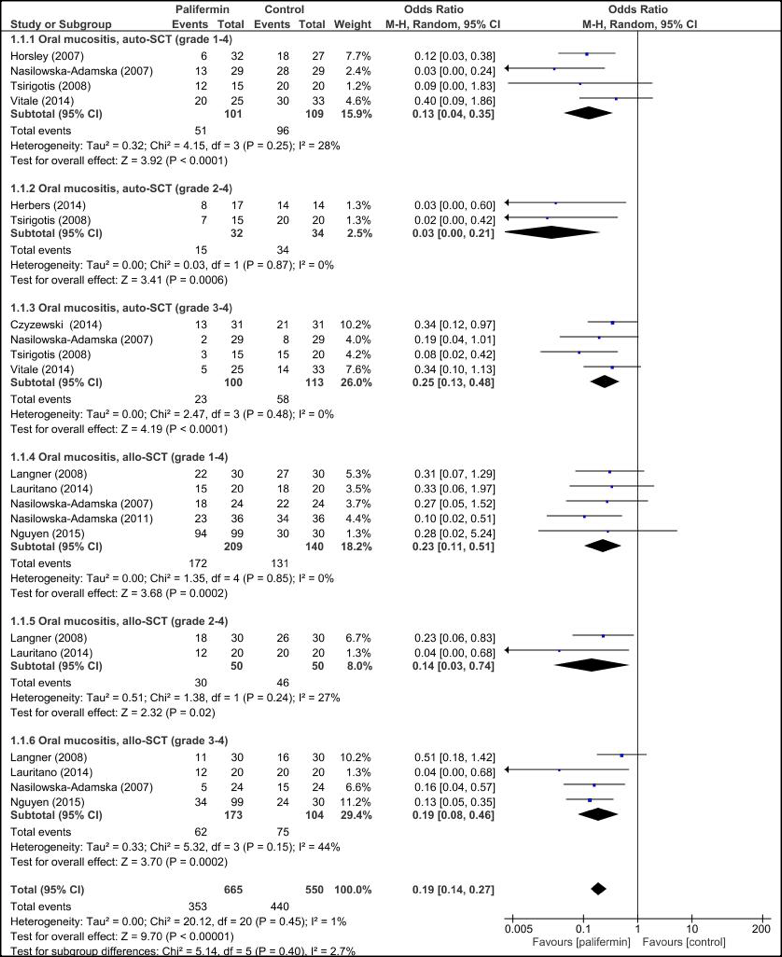
Figure 2 shows OR of OM and aGVHD grades in palifermin group compared with control group after HSCT. The pooled subgroup analysis with dichotomous data demonstrated that palifermin was more effective on OM (grade 1-4), (grade 2-4) and (grade 3-4) with [odds ratio (OR)= 0.17; 95%CI= 0.10,0.29; p <0.00001], [OR= 0.11; 95%CI= 0.05,0.24; p <0.00001] and [OR= 0.22; 95%CI= 0.15,0.33; p <0.00001], respectively, but palifermin was not effective on aGVHD (grade 1-4), (grade 2-4) and (grade 3-4) that [OR= 0.53; 95%CI= 0.26,1.08; p <0.08], [OR= 0.93; 95%CI= 0.43,1.98; p <0.85] and [OR= 0.56; 95%CI= 0.23,1.36; p <0.85] were respectively, without heterogeneity.
Meta-analysis: The incidence of OM based on the type of HSCT
The pooled subgroup analysis demonstrated that palifermin was more effective on OM after auto-HSCT and allo-HSCT ( Figure 3 ). The subgroup analysis showed OM (grade 1-4) had [OR= 0.13; 95%CI= 0.04, 0.35; p <0.0001], (grade 2-4) had [OR= 0.03; 95%CI= 0.00, 0.21; p =0.0006] and (grade 3-4) had [OR= 0.25; 95%CI= 0.13, 0.48; p <0.0001] for auto-HSCT and OM (grade 1-4) had [OR= 0.23; 95%CI= 0.11, 0.51; p =0.0002], (grade 2-4) had [OR= 0.14; 95%CI= 0.03, 0.74; p =0.012] and (grade 3-4) had [OR= 0.19; 95%CI= 0.08, 0.46; p =0.0002] for allo-HSCT. There was no heterogeneity between estimations for all subgroups, exception for allo-HSCT (grade 2-4) and (grade3-4) that was moderate heterogeneity.
Meta-analysis: The incidence of adverse events
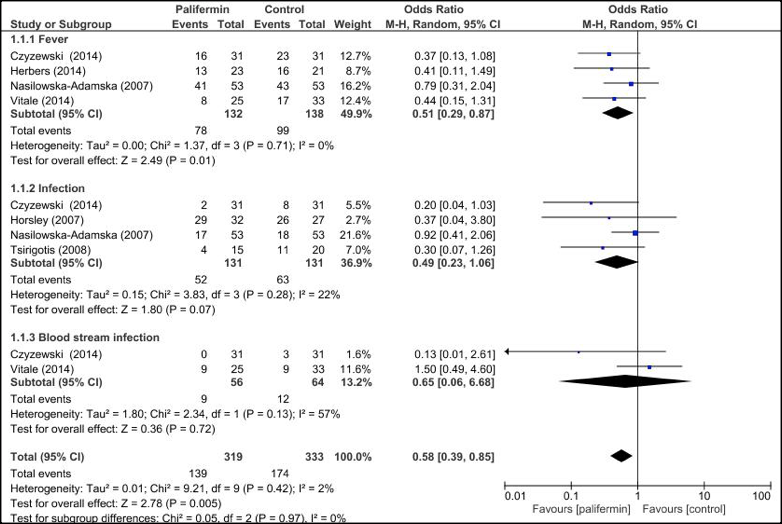
The comparison of frequency of fever (almost>38 °C) and infection has been included in Figure 4 . The pooled subgroup analysis confirmed that the palifermin was more effective on fever [OR=0.51; 95%CI=0.29, 0.87; p = 0.01], but was not for infection [OR=0.49; 95%CI=0.23, 1.06; p = 0.07], without heterogeneity and blood stream infection [OR=0.65; 95%CI= 0.06, 06.68; p = 0.72] with large heterogeneity.
Publication bias
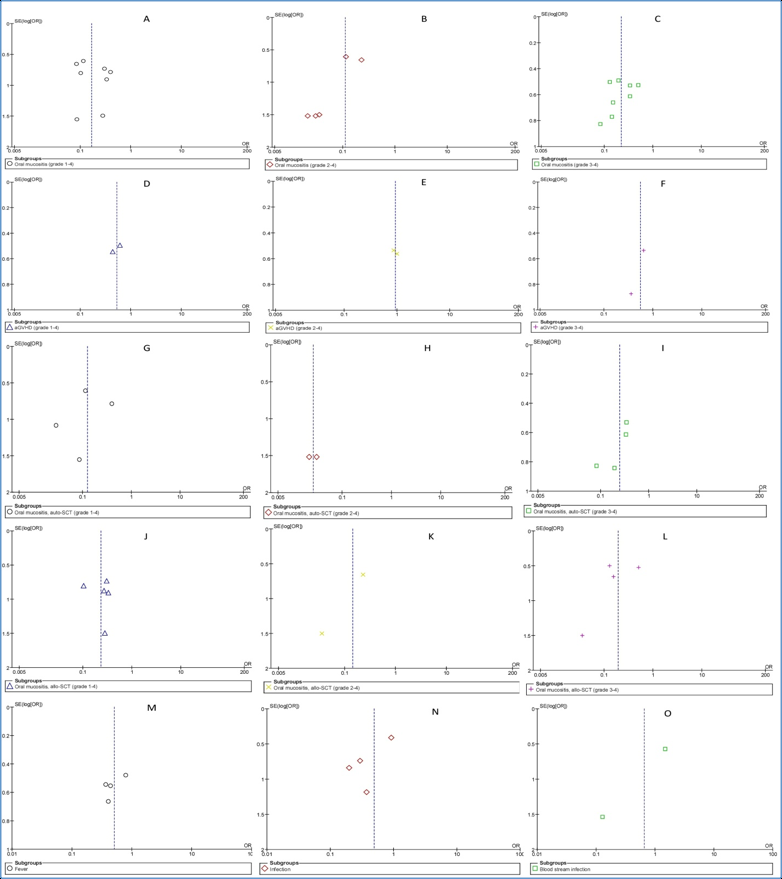
The RevMan 5.3 software was used for publication bias. A funnel plot of each of the pairs of groups compared above was created. If funnel plot was symmetric, it would suggest that the publication bias was minimal and that the results of the present study were credible. The results of the Begg's and Egger's tests revealed that no publication biases existed in terms of oral mucositis (grade 1-4) ( Figure 5A ), oral mucositis (grade 3-4) ( Figure 5C ), oral mucositis (grade 1-4) of auto-HSCT ( Figure 5G ), oral mucositis (grade 3-4) of auto-HSCT ( Figure 5I ), oral mucositis (grade 1-4) of allo-HSCT ( Figure 5J ), oral mucositis (grade 3-4) of allo-HSCT ( Figure 5L ), fever ( Figure 5M ) or infection ( Figure 5N ). Regarding aGVHD grade (1-4) ( Figure 5D ), aGVHD grade (2-4) ( Figure 5E ), aGVHD grade (3-4) ( Figure 5F ), oral mucositis (grade 2-4) of auto-HSCT ( Figure 5H ), oral mucositis (grade 2-4) of allo-HSCT ( Figure 5K ) or blood stream infection ( Figure 5O ), Begg's tests revealed no publication biases, but Egger's tests could not be performed because only two studies were included. Regarding oral mucositis (grade 3-4) ($@fig5B$REFONLY$}), a Begg's test revealed no publication bias, but an Egger's test revealed significant publication bias.
Discussion
This meta-analysis study assessed OM and aGVHD after HSCT in the patients undergoing palifermin therapy compared with the control group. The results showed that palifermin had an effect on reducing the incidence of OM, but not aGVHD. The OM is the most disabling factor in patients with HSCT Stiff et al., 2006. A case-control study Nguyen et al., 2015 reported that palifermin associated with a decrease in the severity of OM and duration of overall OM in patients receiving an allo-HSCT with fractionated total body irradiation (TBI) based conditioning. Also, the administration of palifermin during auto-HSCT in children results in a lower incidence of OM Czyzewski et al., 2014, but a case-control in children evaluated that palifermin could not be recommended as a cure for OM (of any grade) due to the variability in the two groups Kobbe et al., 2010. A randomized-controlled trial by Lucchese et al., 2016 showed a significant reduction in pediatric patients with acute lymphocytic leukemia in the incidence of OM grade 3 and 4 in the palifermin group compared with the control group. There was also a decrease in the degree of severity of OM in the palifermin group. Horsley et al., 2007 concluded a significant reduction in the incidence of severe OM in the standard care group compared to the palifermin group (13 versus 48%) and also a higher incidence of severe OM in the standard care group compared to the palifermin group. A placebo-controlled, double-blind, phase ΙΙΙ trial by Spielberger et al., 2004 reported palifermin reduced the duration and severity of OM after intensive chemotherapy and radiotherapy for hematologic malignancies. It has been confirmed that 60 μg/kg/day for 6 doses of palifermin, can effectively reduce the incidence, severity, and duration of OM and its consequences in TBI- and non-TBI based auto- and allografts without negative influence on transplantation Radtke et al., 2005Spielberger et al., 2004Nasilowska-Adamska et al., 2007Horsley et al., 2007Langner et al., 2008Blazar et al., 2006. A case-control study by Vitale et al. (2014) reported that palifermin prescription did not culminate in a statistically significant decrease in the incidence and grade of OM or the supportive care required following myeloablative auto-HSCT.
Conclusion
Tsirigotis et al. (2008) observed a significant decrease in the incidence and severity of OM in patients treated with keratinocyte growth factor. Palifermin might decrease the incidence, severity, and duration of OM in high-dose methotrexate (HD-MTX)-based treatment Schmidt et al., 2008. This meta-analysis of case-control/matched-control studies showed that palifermin reduced significantly the incidence and severity of OM after HSCT in hematologic malignancy patients.
The safety and efficacy profiles of palifermin were assessed by a case-control study on OM and aGVHD in allo-HSCT. There was no significant difference in the incidence of mild or severe aGVHD grades on day 100 after allo-HSCT Langner et al., 2008 that so Nasilowska-Adamska et al. in a non-randomized and matched-control study Nasilowska-Adamska et al. 2011, confirmed prescription of palifermin doesn’t seem to change the incidence and severity of aGVHD, whereas three other studies indicated that the prescription of palifermin decreases the incidence and severity of aGVHD in allo-HSCT Blazar et al., 2006Krijanovski et al., 1999Panoskaltsis-Mortari et al., 1998. The results of a case-control study suggested that the use of palifermin reduces OM and probably aGVHD after HSCT Nasilowska-Adamska et al., 2007. The severity of regimen-induced OM is dependent on the conditioning regimen and not the source of hematopoietic stem cells; thus, the ability of palifermin to reduce the incidence of severe OM in patients with allo-HSCT is not expected to be different than in patients with auto-HSCT Hensley et al., 2008. Sonis et al. (2004) reported that several factors may affect the incidence of mucositis during HSCT. The most important factors are the diagnosis, type of transplant and type of conditioning regimen. This meta-analysis of case-control studies confirmed that the incidence of OM is the same in auto- and allo-HSCT groups and therefore, type of transplant can’t be an important factor in the severity and incidence of OM. Spielberger et al. (2004) stated that in patients with various hematologic cancers, the incidence of severe mucositis was 98% following conditioning with TBI in combination with high-dose etoposide and cyclophosphamide. In contrast, high-dose melphalan was associated with severe mucositis in 20–45% of patients with multiple myeloma Grazziutti et al., 2006Blijlevens et al., 2008. The rate of severe mucositis increased to 80% when the conditioning regimen was augmented with idarubicin and cyclophosphamide Fenk et al., 2005. Two studies Nguyen et al., 2015Cutler et al., 2005 found the patients who received MTX as part of their GVHD prophylaxis regimen were more to develop severe OM (p<0.05). This meta-analysis showed that palifermin was not effective on the incidence and severity of aGVHD after HSCT in hematologic cancer patients in case-control studies that due to paucity of studies about the efficacy of palifermin on aGVHD, this result can not be precise. Palifermin could also reduce infections, a complication that after high-dose chemotherapy and auto-HSCT may affect 60-90% of all cases and that is the cause of deaths in 2-3% of all treated patients Gil et al., 2007Kolbe et al., 1997Mossad et al., 1996. The results of this meta-analysis showed that palifermin was effective on fever (p = 0.01) and not infection (p>0.05). In case-control/matched-control studies, one study confirmed the result of the meta-analysis about fever, but three studies Czyzewski et al., 2014Nasilowska-Adamska et al., 2007Vitale et al., 2014 were disagree with the result. The use of antibiotics given empirically to HSCT recipients can decrease fever thereby lowering the risk of adverse events and the induction of microbial resistance Herbers et al., 2014. Therefore, antibiotics and topical palliative drugs can help the patients to overcome infections and other unwanted events Dazzi et al., 2003Stiff et al., 2001.
Conclusion
In conclusion, although this meta-analysis (the strength: lack of heterogeneity in more analyses) showed that palifermin was associated with reductions in the incidence and severity of OM and also was effective and safe on OM after allo-or auto-HSCT, but did not seem to affect on the incidence and severity of aGVHD, it had several limitations including the numbers of patients in some studies were relatively small; there were variable types of chemotherapy during HSCT in the studies; the age range, the type of HSCT and malignancy were different in the studies. Therefore, due to the above-mentioned limitations and the contradictions of the combined results, studies of large-scale and well-designed with better exposure evaluations are guaranteed to support the findings of our study and create a higher level of evidence.
Abbreviations
aGVHD: Acute GVHD
CI: Confidence intervals
FDA: Food and Drug Administration
HD-MTX: High-dose methotrexate
HSCT: Hematopoietic Stem Cell Transplantation
OM: Oral mucositis
OR: Odds ratio
TBI: Total body irradiation
WHO: World Health Organization
Author Contributions
Conceptualization: MP MS HRM
Data curation: MR MS
Formal analysis: MR MS
Funding acquisition: MP HRM
Investigation: MP MS
Methodology: MR MS
Project administration: MP HRM
Resources: MR MS
Software: MS
Supervision: MP MR HRM
Validation: MP MR HRM
Visualization: MR MS
Writing – original draft: MS HRM
Writing – review & editing: MR MP MS HRM
References
-
B. R.
Blazar,
D. J.
Weisdorf,
T.
Defor,
A.
Goldman,
T.
Braun,
S.
Silver,
J. L.
Ferrara.
Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) . Blood.
2006;
108(9)
:
3216-3222
.
View Article Google Scholar -
N.
Blijlevens,
M.
Schwenkglenks,
P.
Bacon,
A.
D’Addio,
H.
Einsele,
J.
Maertens,
S.
McCann.
Prospective oral mucositis audit: Oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy—European Blood and Marrow Transplantation Mucositis Advisory Group . Journal of Clinical Oncology.
2008;
26(9)
:
1519-1525
.
View Article Google Scholar -
C.
Cutler,
S.
Li,
H. T.
Kim,
P.
Laglenne,
K. C.
Szeto,
L.
Hoffmeister,
J. H.
Antin.
Mucositis after Allogeneic Hematopoeitic Stem Cell Transplantation: A Cohort Study of Methotrexate- and Non-Methotrexate-Containing Graft-versus-Host Disease Prophylaxis Regimens . Biology of Blood and Marrow Transplantation.
2005;
11(5)
:
383-388
.
View Article Google Scholar -
K.
Czyzewski,
R.
Debski,
A.
Krenska,
M.
Wysocki,
J.
Styczynski.
Palifermin in children undergoing autologous stem cell transplantation: A matched-pair analysis. Anticancer Research.
2014;
34
:
7379-7382
.
-
C.
Dazzi,
A.
Cariello,
P.
Giovanis,
M.
Monti,
B.
Vertogen,
M.
Leoni.
Prophylaxis with GM-CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high-dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: A double blind, randomized, placebocontrolled study . Annals of Oncology : Official Journal of the European Society for Medical.
2003;
Oncology
:
14(4), 559-563
.
View Article Google Scholar -
M.
Egger,
G.
Smith,
M.
Schneider,
C.
Minder.
Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.).
1997;
315(7109)
:
629-634
.
-
J. B.
Epstein,
J.
Thariat,
R. J.
Bensadoun,
A.
Barasch,
B. A.
Murphy,
L.
Kolnick,
E.
Maghami.
Oral complications of cancer and cancer therapy: From cancer treatment to survivorship . Cancer J.
2012;
Clin
:
62(6), 400-422
.
View Article Google Scholar -
R.
Fenk,
P.
Schneider,
M.
Kropff,
A. N.
Huenerlituerkoglu,
U.
Steidl,
C.
Aul,
G.
Kobbe.
High-dose idarubicin, cyclophosphamide and melphalan as conditioning for autologous stem cell transplantation increases treatment-related mortality in patients with multiple myeloma: Results of a randomised study . British Journal of.
2005;
Haematology
:
130(4), 588-594
.
View Article Google Scholar -
J.
Filicko,
H. M.
Lazarus,
N.
Folmenberg.
Mucosal injury in patients undergoing hematopoietic progenitor cell transplantation: New approaches to prophylaxis and treatment . Bone Marrow Transplantation.
2003;
31(1)
:
1-10
.
View Article Google Scholar -
L.
Gil,
G.
Styczynski,
M.
Komarnicki.
Infectious complication In 314 patients after high dose therapy and autologous hematopoietic stem cell transplantation: Risk factor analysis and outcome . Infection.
2007;
35(6)
:
421-427
.
View Article Google Scholar -
M. L.
Grazziutti,
L.
Dong,
M. H.
Miceli,
S. G.
Krishna,
E.
Kiwan,
N.
Syed,
E. J.
Anaissie.
Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: Incidence, risk factors and a severity predictive model . Bone Marrow Transplantation.
2006;
38(7)
:
501-506
.
View Article Google Scholar -
M. L.
Hensley,
K. L.
Hagerty,
T.
Kewalramani,
D. M.
Green,
N. J.
Meropol,
T. H.
Wasserman,
L. M.
Schuchter.
American Society of Clinical Oncology 2008 Clinical Practice Guideline Update: Use of Chemotherapy and Radiation Therapy Protectants . Journal of Clinical Oncology.
2008;
27(1)
:
127-145
.
View Article Google Scholar -
A. H.
Herbers,
W. J.
van der Velden,
A. F.
de Haan,
J. P.
Donnelly,
N. M.
Blijlevens.
Impact of palifermin on intestinal mucositis of HSCT recipients after BEAM . Bone Marrow Transplantation.
2014;
49(1)
:
8-10
.
View Article Google Scholar -
J.
Hołowiecki.
Indications for hematopoietic stem cell transplantation. Polskie Archiwum Medycyny Wewnetrznej.
2008;
118
:
658-663
.
-
P.
Horsley,
J. D.
Bauer,
R.
Mazkowiack,
R.
Gardner,
J.
Bashford.
Palifermin improves severe mucositis, swallowing problems, nutrition impact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation . Supportive Care in Cancer.
2007;
15(1)
:
105-109
.
View Article Google Scholar -
S.
Jilani,
Z.
Kanaan,
R.
Agarwal,
N.
Tageja,
M. H.
Abidi.
Palifermin for Prevention of Oral Mucositis in Hematological Malignancies: Present Position and Future Perspectives. Austin J Cancer Clin Res.
2014;
1
:
1010, null
.
-
O. I.
Krijanovski,
G. R.
Hill,
K. R.
Cooke,
T.
Teshima,
J. M.
Crawford,
Y. S.
Brinson,
J. L.
Ferrara.
Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood.
1999;
94
:
825-831
.
-
G.
Kobbe,
I.
Bruns,
T.
Schroeder,
A.
Czibere,
J.
Warnecke,
N.
Hieronimus,
R.
Fenk.
A 3-day short course of palifermin before HDT reduces toxicity and need for supportive care after autologous blood stem-cell transplantation in patients with multiple myeloma . Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2010;
21(9)
:
1898-1904
.
View Article Google Scholar -
H. J.
Kolb.
Hematopoietic stem cell transplantation and cellular therapy . HLA.
2017;
89(5)
:
267-277
.
View Article Google Scholar -
K.
Kolbe,
D.
Domkin,
H. G.
Derigs,
S.
Bhakdi,
C.
Huber,
W. E.
Aulitzky.
Infectious complications during neutropenia subsequent to peripheral blood stem cell transplantation . Bone Marrow Transplantation.
1997;
19(2)
:
143-147
.
View Article Google Scholar -
S.
Langner,
P.
Staber,
N.
Schub,
M.
Gramatzki,
W.
Grothe,
G.
Behre,
P.
Neumeister.
Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients . Bone Marrow Transplantation.
2008;
42(4)
:
275-279
.
View Article Google Scholar -
D.
Lauritano,
M.
Petruzzi,
D.
Di Stasio,
A.
Lucchese.
Clinical effectiveness of palifermin in prevention and treatment of oral mucositis in children with acute lymphoblastic leukaemia: A case-control study . International Journal of Oral Science.
2014;
6(1)
:
27-30
.
View Article Google Scholar -
A.
Lucchese,
G.
Matarese,
L. H.
Ghislanzoni,
G.
Gastaldi,
M.
Manuelli,
E.
Gherlone.
Efficacy and effects of palifermin for the treatment of oral mucositis in patients affected by acute lymphoblastic leukemia . Leukemia & Lymphoma.
2016;
57(4)
:
820-827
.
View Article Google Scholar -
S. B.
Mossad,
D. L.
Longworth,
M.
Goormastic,
J. M.
Serkey,
T. F.
Keys,
B. J.
Bolwell.
Early infectious complications in autologous bone marrow transplantation: A review of 219 patients. Bone Marrow Transplantation.
1996;
18
:
265-271
.
-
B.
Nasilowska-Adamska,
P.
Rzepecki,
J.
Manko,
A.
Czyz,
M.
Markiewicz,
I.
Federowicz,
B.
Marianska.
The influence of palifermin (Kepivance) on oral mucositis and acute graft versus host disease in patients with hematological diseases undergoing hematopoietic stem cell transplant . Bone Marrow.
2007;
Transplantation
:
40(10), 983-988
.
View Article Google Scholar -
B.
Nasilowska-Adamska,
R.
Szydlo,
P.
Rzepecki,
A.
Czyz,
A.
Tomaszewska,
M.
Markiewicz,
W. W.
Jedrzejczak.
Palifermin does not influence the incidence and severity of GvHD nor long-term survival of patients with hematological diseases undergoing HSCT . Annals of Transplantation.
2011;
16(4)
:
47-54
.
View Article Google Scholar -
D. T.
Nguyen,
S.
Shayani,
J.
Palmer,
A.
Dagis,
S. J.
Forman,
J.
Epstein,
R.
Spielberger.
Palifermin for prevention of oral mucositis in allogeneic hematopoietic stem cell transplantation: A single-institution retrospective evaluation . Supportive Care in Cancer.
2015;
23(11)
:
3141-3147
.
View Article Google Scholar -
A.
Panoskaltsis-Mortari,
D. L.
Lacey,
D. A.
Vallera,
B. R.
Blazar.
Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood.
1998;
92
:
3960-3967
.
-
M. L.
Radtke,
J. M.
Kolesar.
Palifermin (Kepivance) for the treatment of oral mucositis in patients with hematologic malignancies requiring hematopoietic stem cell support . Journal of Oncology Pharmacy Practice.
2005;
11(3)
:
121-125
.
View Article Google Scholar -
E.
Schmidt,
N. H.
Thoennissen,
A.
Rudat,
R.
Bieker,
C.
Schliemann,
R. M.
Mesters,
W. E.
Berdel.
Use of palifermin for the prevention of high-dose methotrexate-induced oral mucositis . Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2008;
19(9)
:
1644-1649
.
View Article Google Scholar -
R.
Spielberger,
P.
Stiff,
W.
Bensinger,
T.
Gentile,
D.
Weisdorf,
T.
Kewalramani,
C.
Emmanouilides.
Palifermin for oral mucositis after intensive therapy for hematologic cancers . The New England Journal of.
2004;
Medicine
:
351(25), 2590-2598
.
View Article Google Scholar -
S.
Sonis,
J.
Clark.
Prevention and management of oral mucositis induced by antineoplastic therapy. Oncology (Williston Park, N.Y.).
1991;
5
:
11-18
.
-
S. T.
Sonis,
L. S.
Elting,
D.
Keefe,
D. E.
Peterson,
M.
Schubert,
M.
Hauer-Jensen,
E. B.
Rubenstein.
Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients . Cancer.
2004;
100(S9)
:
1995-2025
.
View Article Google Scholar -
P. J.
Stiff,
H.
Erder,
W. I.
Bensinger,
C.
Emmanouilides,
T.
Gentile,
J.
Isitt,
R.
Spielberger.
Reliability and validity of a patient selfadministered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT) . Bone Marrow Transplantation.
2006;
37(4)
:
393-401
.
View Article Google Scholar -
P.
Stiff.
Mucositis associated with stem cell transplantation: Current status and innovative approaches to management . Bone Marrow Transplantation.
2001;
27
:
S3-S11
.
View Article Google Scholar -
P.
Tsirigotis,
K.
Triantafyllou,
K.
Girkas,
V.
Giannopoulou,
E.
Ioannidou,
S.
Chondropoulos,
J.
Dervenoulas.
Keratinocyte growth factor is effective in the prevention of intestinal mucositis in patients with hematological malignancies treated with high-dose chemotherapy and autologous hematopoietic SCT: A video-capsule endoscopy study . Bone Marrow Transplantation.
2008;
42(5)
:
337-343
.
View Article Google Scholar -
K. M.
Vitale,
L.
Violago,
P.
Cofnas,
J.
Bishop,
Z.
Jin,
M.
Bhatia,
P.
Satwani.
Impact of palifermin on incidence of oral mucositis and healthcare utilization in children undergoing autologous hematopoietic stem cell transplantation for malignant diseases . Pediatric Transplantation.
2014;
18(2)
:
211-216
.
View Article Google Scholar -
S. B.
Woo,
S. T.
Sonis,
M. M.
Monopoli,
A. L.
Sonis.
A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients . Cancer.
1993;
72(5)
:
1612-1617
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 10 (2017)
Page No.: 1676-1692
Published on: 2017-10-14
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8412 times
- Download PDF downloaded - 2458 times
- View Article downloaded - 11 times
 Biomedpress
Biomedpress