Incidence and Mortality of Liver Cancer and their Relationship with the Human Development Index in the World
Abstract
Background: Information on the incidence and mortality of liver cancer can be useful for health programs and research activities, and with regard to the possible role of the HDI and liver cancer, this study aimed to investigate incidence and mortality from the cancer and their relationship with the indicator and its components in 2012 in world.
Methods: In this ecologic study, data were extracted from GLOBOCAN in 2012. Data on HDI and its components were extracted from the World Bank. The number and standardized incidence and mortality rates of liver cancer were reported by regions in the world. Data were analyzed by SPSS software and Correlation coefficient test.
Results: There was a total of 782,451 incidence cases. Of these patients, 228, 082 cases (29.15 %) were women and 554, 369 cases (70.85 %) men, and there were 745,533 deaths. Of the deaths, 224, 492 cases (30.11 %) occurred in women and 521, 041 cases (69.89 %) men were recorded in the world in 2012. Results showed that there was a significant inverse correlation between age-specific incidence rate ( ASIR) and HDI (r=-0.345, p≤0.001), as well significant inverse correlation was seen between age-specific mortality rate (ASMR), and HDI and its components.
Conclusion: Liver cancer incidence and mortality are higher in the medium HDI countries. The relationship between the standardized incidence and mortality of liver cancer with HDI and its components, including life expectancy at birth, mean years of schooling and income level per person was significantly negative.
Introduction
Hepatocellular carcinoma (HCC) is one of the main causes of mortality worldwide and most cases occur in Asia and Africa . It is the fifth and seventh cause of death from cancer in men and women, respectively Shen et al., 2012.
The cancer is classified into three types of intrahepatic cholangiocarcinoma (ICC), HCC and the combination of the two classifications Ikai et al., 2004Tang et al., 2006. HCC, as main form of primary cancer, is an aggressive malignancy and more than 50% of cases died within the first year after diagnosis Altekruse et al., 2009Jemal et al.,2012. Evidence suggests that HCC incidence is still increasing (Kim et al., 2014). Increased trends in rates of PLC for both sexes have been observed in many populations, for example: Europe, America, Oceania, the United Kingdom, France, Germany, Netherlands, Italy, Canada and Colombia Zhang et al., 2015.
The highest increasing trend of DALY was attributed to pancreatic and liver cancers between 1990 and 2010 Murray et al., 2013. In 2013, among US adults, 22720 men and 7920 women were diagnosed with primary liver cancer, which is increasing Siegel et al., 2013. There is a high prevalence of liver cancer in 22 countries, mainly in East Africa and Southeast Asia, as a result of risk factors in these areas Bray et al., 2012. It was seen that 25% of cancers related to infectious agents (liver, stomach and cervix cancers) occur in sub-Saharan Africa while 27% of cases were seen in East Asia, and incidence of liver cancer in Asian countries is increasing almost two times Soerjomataram et al., 2012. International trends in primary liver cancer incidence rates during 1973 to 2007 revealed that most ESR for primary liver cancer was a range from 19 to 26.7 per 100,000 men and from 4.8 to 8.7 per 100,000 women in some Asian populations Zhang et al., 2015. HCG differences in the incidence rates in different geographical areas are correlated with hepatitis B and C rates because chronic viral hepatitis has been reported as an important risk factor for HCC in endemic areas Chisari et al., 1989Colombo et al., 1989Idilman et al., 1998. This is despite the fact that approximately 45 million and 15 million people worldwide suffer from chronic hepatitis B and C Kar, 2014. Another reason for the difference in incidence in different regions was Human papillomavirus (HPV), which is responsible for stomach, liver and cervical cancers Vineis and Wild, 2014. Other etiologies for liver cancer are modifiable risk factors, such as smoking, alcohol, contaminated injections in health care centers, immigration and obesity Chuang et al., 2009Ezzati and Riboli, 2013Franceschi and Wild, 2013Shih et al., 2012. The relative risk associated with family history of liver cancer is 2.6 and the cancer is also significantly more common in men Huang et al., 2011Yang et al., 2014.
The difference in the incidence of deaths from cancer between different countries can be caused by differences in development of countries. Some studies have confirmed the relationship between human development indicators (HDI) with the incidence and mortality of cancer Bray et al., 2012Rafiemanesh et al., 2016.
In countries with a low HDI, incidence and mortality rates from cervical, liver, esophagus and stomach cancers are higher than countries with high HDI Franceschi and Bray, 2014Humans, 2011Pakzad et al., 2016. Also, studies showed that some of cancer is related to the HDI Ghoncheh et al., 2015Pakzad et al., 2015a, b. Chronic diseases, including liver cancer, have multiple causes, and one of the possible causes could be development. Therefore, having information on the incidence and mortality of liver cancer can be useful for health programs and research activities and with regard to the possible role of the HDI, this study aimed to investigate incidence and mortality from the cancer and their relationship with the indicator and its components in 2012 in world.
Methods
In this ecologic study, data about the age-specific incidence and mortality rate (ASR) for every country in 2012 were get from the global cancer project that available in (http://globocan.iarc.fr/Default.aspx) Ferlay J et al., 2016 and HDI from Human Development Report 2013 Malik, 2013, that includes information about HDI and its details for every country in the word in 2012. More details about data collection were previously published Ferlay J et al., 2016Ferlay et al., 2015.
Method for estimating the age-specific incidence and mortality rates in global cancer project by international agency for research on cancer
Age-specific incidence rate estimate
The methods of estimation are country specific, and the quality of the estimation depends upon the quality and on the amount of the information available for each country. In theory, there are as many methods as countries, and because of the variety and the complexity of these methods, an overall quality score for the incidence and mortality estimates combined is almost impossible to establish. However, an alphanumeric scoring system which independently describes the availability of incidence and mortality data has been established at the country level. The combined score is presented together with the estimates for each country with an aim of providing a broad indication of the robustness of the estimation. The methods to estimate the sex- and age-specific incidence rates of cancer for a specific country fall into one of the following broad categories, in priority order:
1. Rates projected to 2012 (38 countries)
2. Most recent rates applied to population in 2012 (20 countries)
3. Estimated from national mortality by modelling, using incidence mortality ratios derived from recorded data in country-specific cancer registries (13 countries)
4. Estimated from national mortality estimates by modelling, using incidence mortality ratios derived from recorded data in local cancer registries in neighboring countries (9 European countries)
5. Estimated from national mortality estimates using modelled survival (32 countries)
6. Estimated as the weighted average of the local rates (16 countries)
7. One cancer registry covering a part of a country is used as representative of the country profile (11 countries)
8. Age/sex specific rates for "all cancers" were partitioned using data on relative frequency of different cancers (by age and sex) (12 countries)
9. The rates are those of neighboring countries or registries in the same area (33 countries) Ferlay J et al., 2016.
Age-specific mortality rate estimate
Depending on the degree of detail and accuracy of the national mortality data, six methods have been utilized in the following order of priority:
1. Rates projected to 2012 (69 countries)
2. Most recent rates applied to population in 2012 (26 countries)
3. Estimated as the weighted average of regional rates (1 country)
4. Estimated from national incidence estimates by modelling, using country-specific survival (2 countries)
5. Estimated from national incidence estimates using modelled survival (83 countries)
6. The rates are those of neighboring countries or registries in the same area (3 countries) Ferlay J et al., 2016Ferlay et al., 2015.
For assessment of the correlation between age-specific incidence and mortality rate (ASR) with HDI and its details, which include life expectancy at birth, mean years of schooling and gross national income (GNI) per capita, we used correlation bivariate method. Statistical significance was assumed if P<0.05. Statistical analyses were performed using SPSS (Version 15.0, SPSS Inc.).
Results
Incidence rate
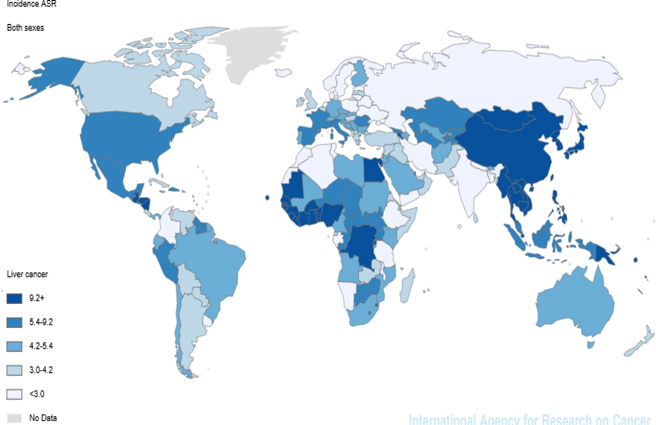
In 2012, 782,451 new patients suffered from the cancer were diagnosed; so that the patients included 5.6% of all cancers. Of these patients, 228, 082 cases (29.15 %) were women and 554, 369 cases (70.85 %) men. According to the estimates of GLOBOCAN, the cancer with ASIR equal to 10.1 per 100,000 is known as the seventh common cancer in the world. The cancer is the ninth and fifth common cancer in women and men with the incidence of 5.3 and 15.3, respectively. Of the six WHO regions, the highest incidence rate was seen in the region WPRO (20.4) and the lowest incidence rate was 4.3 for the region EUROP. Considering the regions in terms of development (HDI), the highest incidence rate (14.6) belonged to Medium Human Development and the lowest incidence rate (3.8) to High Human Development. Among the countries in the world, the highest and lowest incidence rates were related to the countries MONGOLIA and NEPAL, 78.1 and 0.9 respectively. According to the division of the continent, the highest incidence rate was taken in account to Eastern Asia and Northern Europe, 20.9 and 3.1 respectively ( Figure 1 ).
Mortality rate
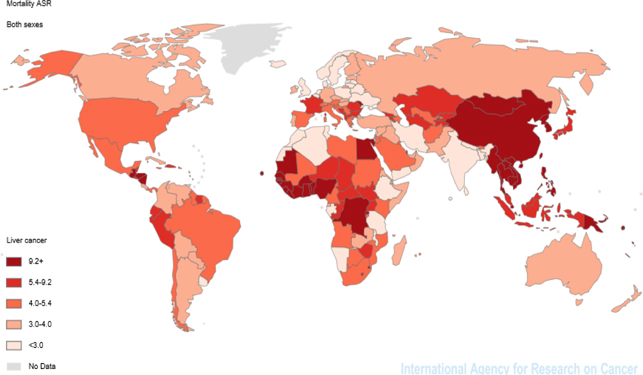
It was estimated that there were 745,533 deaths from the cancer in 2012. Of the deaths, 224, 492 cases (30.11 %) occurred in women and 521, 041 cases (69.89 %) men. The cancer with ASMR equal to 9.5 per 100,000 is the third cause of death worldwide. Liver cancer in women with mortality rate of 5.1 was the sixth cause of cancer death after breast, lung, and cervix uteri cancers and in men with mortality rate of 14.3 was second common and fatal cancer after lung cancer. Of the six WHO regions, the highest mortality rate was seen in the region WPRO (19.1) and the lowest mortality rate was 4 for the region EUROP. Considering the regions in terms of development (HDI), the highest mortality rate (14.1) was observed in Medium Human Development and the lowest incidence rate (4.1) in High Human Development. According to the division of the continent, the highest mortality rates were related to Eastern Asia and Northern Europe, 19.6 and 2.8 respectively ( Figure 2 ).
HDI
The ratio of mortality to incidence (MIR) was estimated about 0.95 in the world for the cancer. This ratio for the six WHO regions was different from 1.07 for EUROP and WPRO to 1.13 for PAHO and 1.04 for AFRO, SEARO and EMRO. Considering the regions in terms of development (HDI), the highest ASMR (69%) was observed in Medium Human Development and the lowest ASMR (6.9%) in High Human Development.
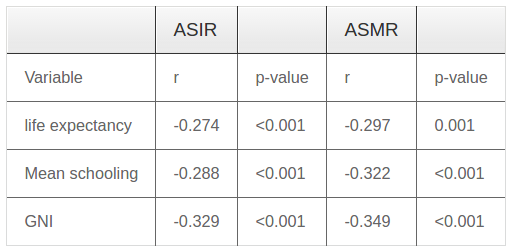
Results showed that there was a significant inverse correlation between ASIR and HDI (r=-0.345, p≤0.001). Pearson correlation test revealed significant correlation between ASIR and components of the HDI, including life expectancy at birth, mean years of schooling and Gross national product (GNI) ( Table 1 ). Significant inverse correlation was seen between ASMR and HDI and its components ( Table 1 ).
Discussion
Liver cancer was the fifth common cancer in the world with 437,000 new cases in 1990 and the incidence rate was 2 to 3 times higher in developing countries than in developed countries El-Serag, 2012. However, in 2014 the cancer was dropped to seventh common cancer in the world with 782,451 new cases and more than 85% of cases of primary liver cancer are diagnosed in developing countries. Highest incidence rate is attributed to regions of Southeast Asia and Sub-Sabhan Africa El-Serag, 2012El-Serag and Rudolph, 2007.
Based on the results of this study, the highest and lowest rates of cancer incidence and mortality were seen in the West Pacific, East Asia and North Europe, respectively; but both of these rates are negatively correlated with the development index and its components. Polk DB in 2010 and Perz JF in 2006 found that stomach cancer and liver cancer are common in areas with medium HDI Perz et al., 2006Polk and Peek,2010. Milena Maule (2012) indicated that although absolute risk of cancer is down in countries with low HDI, some cancers, such as cervical, stomach, liverand Kaposi’s sarcoma, have the highest incidence and mortality rates in these areas Franceschi and Wild, 2013Maule and Merletti, 2012. Etiology of these cancers is resulting from infectious agents. In a study by El-Serag HB in 2004, it was found that liver cancer in Asian countries is on the rise 2 time El-Serag, 2004. Freddie Bray in 2012 also indicated that there is a high prevalence of liver cancer in 22 countries, mainly in East Africa and Southeast Asia Bray et al., 2012. An investigation on all cancers registered between 2008 and 2013 in regions with different HDI showed that the annual incidence increased to 75 % Bray et al., 2012. Increasing trend from 1990 to 2014 revealed that the incidence is higher in developing countries and is consistent with the results of this study. Yue Zhang in 2015 did not confirm the result of this study and showed that there were the declining trend in Asian populations and the increasing trend in some European and Pacific populations between 1973 and 2007 Zhang et al., 2015. According to final results of HCC in the United States during 2007 to 2010, there was no significant increase in incidence, but mortality rate has increased Altekruse et al.,2014. A study by Silvia Franceschi in 2015 it was shown that an epidemiological transition towards patterns of cancers associated with infectious agents in countries with a high HDI Franceschi and Wild, 2013. Although the reasons for this trend are completely not clear, it seems that public health measures in Asia and HCV transmission in Europe, America, and Oceania contributed to the formation of these patterns Zhang et al., 2015. The stunning result of this study was that the maximum and minimum MIR by the division of the continent was 1.25 and 0.76 for Central America and Northern America. Qi-Da Hu in 2013 indicated that an inverse relationship between HDI and MIR, which is contrary to findings of the present study Altekruse et al., 2009. This difference may be due to registration of the death rates in many countries and diagnostic accuracy of liver cancer cases in health care systems. F. Vostakolaei (2010) confirmed the reasons. On the other hand, the prognosis for primary liver cancer is poor, and the cancer is typically diagnosed in advanced stages Vostakolaei et al., 2011. Therefore, the difference in reporting can have remarkable impact on MIR.
In this study, sex ratio of ASIR (male to female) was 2.89 around the world. Bosch FX in 1999 the sex ratio of ASIR was 1.5 to 3, which is consistent with the present study Bosch et al., 1999. Of the cancers related to infectious agents, about half of the burden of cervical cancer was seen in women, while more than 80 % of liver cancer and stomach occurred in men Vineis and Wild, 2014. The high incidence of liver cancer in men can be at higher risk of the most important factors such as smoking, alcohol, and infected injection Franceschi and Wild, 2013Kar, 2014. In examining the relationship between the life expectancy of the HDI, an inverse relationship was observed between the component with rates of incidence and mortality. Given that the age is a firmprognosis in many cancers, age may have a paradoxical role in relation to cancer prognosis Chen et al., 2006. Liver cancer is one of cancers that influenced by the paradoxical role of age because about 30 % of cancers related to infection agents occur in people younger than 50 years Vineis and Wild, 2014. Although liver cancer is more common in people 65 years, the incidence and mortality of the cancer are higher in younger age because the main risk factors for liver cancer, such as viral hepatitis and alcohol abuse, affect people in early middle age Blachier et al., 2013Starr and Raines, 2011.
In this study, an inverse and significant relationship was observed between national income with rates of incidence and mortality from the cancer. Ahmedin Jemal confirmed that HCC cases in each ethnic group had the lowest socio-economic status compared with their reference population Jemal et al., 2013. Other studies have shown an inverse relationship between low socioeconomic and the incidence of HCC Karlamangla et al., 2010. HB antigen prevalence and alcohol abuse and finallyliver cancer are higher in people who have low socioeconomic status because the persons more exposed to risk factors and have poor access to health care system. As a result, higher incidence and mortality rates were observed among them Akil and Ahmad,2011Joshi et al., 2008Karlamangla et al., 2010Khan et al., 2002Loucks et al.,2007Roblin et al., 2010.
In examining the relationship between education level of HDI with the incidence and mortality of the cancer, significantly negative correlation was seen.Ahmedin Jemal revealed that an increase in death rates for liver cancer is common in people with lower degrees of education Jemal et al., 2013. In Flores YN’s study, it was found that if the level of education is lower, viral load of hepatitis infection and the incidence of HCC will be more Flores et al., 2008. Kyunghee Jung-Choi indicated that mortality from the cancer is higher in population groups with lower education than population groups with higher education Jung-Choi et al., 2014. Li-Yen GOH also confirmed that drinking alcohol is effective in increased risk of incidence and mortality from liver cancer in men with lower levels of education. Therefore, the low education level is associated with increased risk factors for the cancer GOH et al., 2014.
Conclusion
Liver cancer incidence and mortality are higher in the medium HDI countries.Therefore, it seems that health policy makers should make serious decisions in this area to deal with the increase in the incidence and mortality of Liver cancer.The relationship between the standardized incidence and mortality of liver cancer with HDI and its components, including life expectancy at birth, mean years of schooling and income level per person, was significantly negative.
Plans for the control and prevention of this cancer must be a high priority for health policy makers; also, it is necessary to increase awareness of risk factors and early detection in all countries, also it is necessary to investigate the related risk factors in medium HDI countries and less developed countries.
References
-
L.
Akil,
H.A.
Ahmad.
Effects of socioeconomic factors on obesity rates in four southern states and Colorado. Ethnicity & disease.
2011;
21
:
58
.
-
S.F.
Altekruse,
S.J.
Henley,
J.E.
Cucinelli,
K.A.
McGlynn.
Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. he American journal of gastroenterology.
2014;
109
:
542-553
.
-
S.F.
Altekruse,
K.A.
McGlynn,
M.E.
Reichman.
Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of clinical oncology.
2009;
27
:
1485-1491
.
-
M.
Blachier,
H.
Leleu,
M.
Peck-Radosavljevic,
D.-C.
Valla,
F.
Roudot-Thoraval.
The burden of liver disease in Europe: a review of available epidemiological data. ournal of hepatology.
2013;
58
:
593-608
.
-
F.X.
Bosch,
J.
Ribes,
J.
Borras.
Epidemiology of primary liver cancer. Semin Liver Dis.
1999;
19
:
271-285
.
-
F.
Bray,
A.
Jemal,
N.
Grey,
J.
Ferlay,
D.
Forman.
Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. The lancet oncology.
2012;
13
:
790-801
.
-
C.H.
Chen,
T.T.
Chang,
K.S.
Cheng,
W.W.
Su,
S.S.
Yang,
H.H.
Lin,
S.S.
Wu,
C.M.
Lee,
C.S.
Changchien,
C.J.
Chen.
Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver international.
2006;
26
:
766-773
.
-
F.V.
Chisari,
K.
Klopchin,
T.
Moriyama,
C.
Pasquinelli,
H.A.
Dunsford,
S.
Sell,
C.A.
Pinkert,
R.L.
Brinster,
R.D.
Palmiter.
Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell.
1989;
59
:
1145-1156
.
-
S.-C.
Chuang,
C.
La Vecchia,
P.
Boffetta.
Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer letters.
2009;
286
:
9-14
.
-
M.
Colombo,
Q.
Choo,
E.
Del Ninno,
N.
Dioguardi,
G.
Kuo,
M.
Donato,
M.
Tommasini,
M.
Houghton.
Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. The Lancet.
1989;
334
:
1006-1008
.
-
H.B.
El-Serag.
Hepatocellular carcinoma: recent trends in the United States. astroenterology.
2004;
127
:
S27-34
.
-
H.B.
El-Serag.
Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology.
2012;
142, 1264-1273
:
e1261
.
-
H.B.
El-Serag,
K.L.
Rudolph.
Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology.
2007;
132
:
2557-2576
.
-
M.
Ezzati,
E.
Riboli.
Behavioral and dietary risk factors for noncommunicable diseases. New England Journal of Medicine.
2013;
369
:
954-964
.
-
Soerjomataram I
Ferlay J.
GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 2/2/2016.
2016
.
-
J.
Ferlay,
I.
Soerjomataram,
R.
Dikshit,
S.
Eser,
C.
Mathers,
M.
Rebelo,
D.M.
Parkin,
D.
Forman,
F.
Bray.
Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer.
2015;
136
:
E359-386
.
-
Y.N.
Flores,
H.F.
Yee,
M.
Leng,
J.J.
Escarce,
R.
Bastani,
J.
Salmerón,
L.S.
Morales.
Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999-2004. The American journal of gastroenterology.
2008;
103
:
2231-2238
.
-
S.
Franceschi,
F.
Bray.
Chronic conditions rising in low-and middle-income countries: the case of cancer control. Cancer Control.
2014;
17
.
-
S.
Franceschi,
C.P.
Wild.
Meeting the global demands of epidemiologic transition-The indispensable role of cancer prevention. Molecular oncology.
2013;
7
:
1-13
.
-
M.
Ghoncheh,
A.
Mohammadian-Hafshejani,
H.
Salehiniya.
Incidence and Mortality of Breast Cancer and their Relationship to Development in Asia. Asian Pac J Cancer Prev.
2015;
16
:
6081-6087
.
-
L.Y.
GOH,
A.H.R.
Leow,
K.L.
GOH.
Observations on the epidemiology of gastrointestinal and liver cancers in the Asia-Pacific region. Journal of digestive.
2014;
diseases15
:
463-468
.
-
Y.-T.
Huang,
C.-L.
Jen,
H.-I.
Yang,
M.-H.
Lee,
J.
Su,
S.- N.
Lu,
U.H.
Iloeje,
C.-J.
Chen.
Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. Journal of Clinical Oncology.
2011;
29
:
3643-3650
.
-
I.W.G.o.t.E.o.C.R.t.
Humans.
IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.. A Review of Human Carcinogens.
2011;
Vol 100. Part B: Biological Agents
.
-
R.
Idilman,
N.
De Maria,
A.
Colantoni,
D.
Van Thiel.
Pathogenesis of hepatitis B and C‐induced hepatocellular carcinoma. Journal of viral hepatitis.
1998;
5
:
285-299
.
-
I.
Ikai,
Y.
Itai,
K.
Okita,
M.
Omata,
M.
Kojiro,
K.
Kobayashi,
Y.
Nakanuma,
S.
Futagawa,
M.
Makuuchi,
Y.
Yamaoka.
Report of the 15th follow-up survey of primary liver cancer. Hepatology research.
2004;
28
:
21-29
.
-
A.
Jemal,
F.
Bray,
D.
Forman,
M.
O’Brien,
J.
Ferlay,
M.
Center,
D.M.
Parkin.
Cancer burden in Africa and opportunities for prevention. Cancer.
2012;
118
:
4372-4384
.
-
A.
Jemal,
E.P.
Simard,
J.
Xu,
J.
Ma,
R.N.
Anderson.
Selected cancers with increasing mortality rates by educational attainment in 26 states in the United States, 1993-2007. Cancer Causes & Control.
2013;
24
:
559-565
.
-
S.
Joshi,
Y.-M.
Song,
T.-H.
Kim,
S.-I.
Cho.
Socioeconomic status and the risk of liver cancer mortality: a prospective study in Korean men. Public health.
2008;
122
:
1144-1151
.
-
K.
Jung-Choi,
Y.-H.
Khang,
H.-J.
Cho,
S.-C.
Yun.
Decomposition of educational differences in life expectancy by age and causes of death among South Korean adults. BMC public health.
2014;
14
:
560
.
-
P.
Kar.
Risk factors for hepatocellular carcinoma in India. Journal of clinical and experimental hepatology.
2014;
4
:
S34-S42
.
-
A.S.
Karlamangla,
S.S.
Merkin,
E.M.
Crimmins,
T.E.
Seeman.
Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001-2006. Annals of epidemiology.
2010;
20
:
617-628
.
-
S.
Khan,
R.P.
Murray,
G.E.
Barnes.
A structural equation model of the effect of poverty and unemployment on alcohol abuse. Addictive behaviors.
2002;
27
:
405-423
.
-
S.S.
Kim,
J.C.
Hwang,
S.G.
Lim,
S.J.
Ahn,
J.Y.
Cheong,
S.W.
Cho.
Effect of virological response to entecavir on the development of hepatocellular carcinoma in hepatitis B viral cirrhotic patients: comparison between compensated and decompensated cirrhosis. The American journal of gastroenterology.
2014;
109
:
1223-1233
.
-
E.B.
Loucks,
D.H.
Rehkopf,
R.C.
Thurston,
I.
Kawachi.
Socioeconomic disparities in metabolic syndrome differ by gender: evidence from NHANES III. Annals of epidemiology.
2007;
17
:
19-26
.
-
K.
Malik.
Human development report 2013. The rise of the south: Human progress in a diverse world. The Rise of the South: Human Progress in a Diverse World (March 15, 2013). UNDP-HDRO Human Development Reports.
2013
.
-
M.
Maule,
F.
Merletti.
Cancer transition and priorities for cancer control. The lancet oncology.
2012;
13
:
745-746
.
-
C.J.
Murray,
T.
Vos,
R.
Lozano,
M.
Naghavi,
A.D.
Flaxman,
C.
Michaud,
M.
Ezzati,
K.
Shibuya,
J.A.
Salomon,
S.
Abdalla.
Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. The lancet.
2013;
380
:
2197-2223
.
-
R.
Pakzad,
A.
Mohammadian-Hafshejani,
M.
Ghoncheh,
I.
Pakzad,
H.
Salehiniya.
The incidence and mortality of lung cancer and their relationship to development in Asia. Transl Lung Cancer Res.
2015a;
4
:
763-774
.
-
R.
Pakzad,
A.
Mohammadian-Hafshejani,
M.
Ghoncheh,
I.
Pakzad,
H.
Salehiniya.
The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate Int.
2015b;
3
:
135-140
.
-
R.
Pakzad,
A.
Mohammadian-Hafshejani,
B.
Khosravi,
S.
Soltani,
I.
Pakzad,
M.
Mohammadian,
H.
Salehiniya,
Z.
Momenimovahed.
The incidence and mortality of esophageal cancer and their relationship to development in Asia. Ann Transl Med.
2016;
4
:
29
.
-
J.F.
Perz,
G.L.
Armstrong,
L.A.
Farrington,
Y.J.
Hutin,
B.P.
Bell.
The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol.
2006;
45
:
529-538
.
-
D.B.
Polk,
R.M. Jr.
Peek.
Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer.
2010;
10
:
403-414
.
-
H.
Rafiemanesh,
M.
Mehtarpour,
F.
Khani,
S.M.
Hesami,
R.
Shamlou,
F.
Towhidi,
H.
Salehiniya,
B.R.
Makhsosi,
A.
Moini.
Epidemiology, incidence and mortality of lung cancer and their relationship with the development index in the world. ournal of Thoracic Disease.
2016;
8
:
1094-1102
.
-
D.W.
Roblin,
B.D.
Smith,
C.M.
Weinbaum,
M.E.
Sabin.
HCV screening practices and prevalence in an MCO, 2000-2007. The American journal of managed.
2010;
care17
:
548-555
.
-
Q.
Shen,
J.
Fan,
X.-R.
Yang,
Y.
Tan,
W.
Zhao,
Y.
Xu,
N.
Wang,
Y.
Niu,
Z.
Wu,
J.
Zhou.
Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. The lancet oncology.
2012;
13
:
817-826
.
-
W.L.
Shih,
H.C.
Chang,
Y.F.
Liaw,
S.M.
Lin,
S.D.
Lee,
P.J.
Chen,
C.J.
Liu,
C.L.
Lin,
M.W.
Yu.
Influences of tobacco and alcohol use on hepatocellular carcinoma survival. International Journal of Cancer.
2012;
131
:
2612-2621
.
-
R.
Siegel,
D.
Naishadham,
A.
Jemal.
Cancer statistics, 2013. CA: a cancer journal for clinicians.
2013;
63
:
11-30
.
-
I.
Soerjomataram,
J.
Lortet-Tieulent,
D.M.
Parkin,
J.
Ferlay,
C.
Mathers,
D.
Forman,
F.
Bray.
Global burden of cancer in 2008: a systematic analysis of disability-adjusted lifeyears in 12 world regions. The Lancet.
2012;
380
:
1840-1850
.
-
S.P.
Starr,
D.
Raines.
Cirrhosis: diagnosis, management, and prevention. Am Fam Physician.
2011;
84
:
1353-1359
.
-
D.
Tang,
H.
Nagano,
M.
Nakamura,
H.
Wada,
S.
Marubashi,
A.
Miyamoto,
Y.
Takeda,
K.
Umeshita,
K.
Dono,
M.
Monden.
Clinical and pathological features of Allen’s type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. Journal of gastrointestinal surgery.
2006;
10
:
987-998
.
-
P.
Vineis,
C.P.
Wild.
Global cancer patterns: causes and prevention. The Lancet.
2014;
383
:
549-557
.
-
F.A.
Vostakolaei,
H.E.
Karim-Kos,
M.L.
Janssen-Heijnen,
O.
Visser,
A.L.
Verbeek,
L.A.
Kiemeney.
The validity of the mortality to incidence ratio as a proxy for sitespecific cancer survival. The European Journal of Public Health.
2011;
21
:
573-577
.
-
Y.
Yang,
Q.J.
Wu,
L.
Xie,
W.H.
Chow,
N.
Rothman,
H.L.
Li,
Y.T.
Gao,
W.
Zheng,
X.O.
Shu,
Y.B.
Xiang.
Prospective cohort studies of association between family history of liver cancer and risk of liver cancer. International Journal of Cancer.
2014;
135
:
1605-1614
.
-
Y.
Zhang,
J.-S.
Ren,
J.-F.
Shi,
N.
Li,
Y.-T.
Wang,
C.
Qu,
Y.
Zhang,
M.
Dai.
International trends in primary liver cancer incidence from 1973 to 2007. BMC cancer.
2015;
15
:
1
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 09 (2016)
Page No.: 800-807
Published on: 2016-09-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 9324 times
- Download PDF downloaded - 2092 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress