Breast cancer tumor growth is efficiently inhibited by dendritic cell transfusion in murine model
Abstract
The ability of dendritic cells to efficiently present tumor-derived antigens when primed with tumor cell lysates makes them attractive as an approach for cancer treatment. This study aimed to evaluate the effects of dendritic cell transfusion dose on breast cancer tumor growth in a murine model. Dendritic cells were produced from allogeneic bone marrow-derived mononuclear cells that were cultured in RPMI 1640 medium supplemented with 20 ng/mL GMCSF and 20 ng/mL IL-4 for 7 days. These cells were checked for maturation before being primed with a cancer cell-derived antigen. Cancer cell antigens were produced by a rapid freeze-thaw procedure using a 4T1 cell line. Immature dendritic cells were loaded with 4T1 cell-derived antigens. Dendritic cells were transfused into mice bearing tumors at three different doses, included 5.104, 105, and 106 cells/mouse with a control consisting of RPMI 1640 media alone. The results showed that dendritic cell therapy inhibited breast cancer tumors in a murine model; however, this effect depended on dendritic cell dose. After 17 days, in the treated groups, tumor size decreased by 43%, 50%, and 87.5% for the doses of 5 × 104, 105, and 106 dendritic cells, respectively, while tumor size in the control group decreased by 44%. This result demonstrated that dendritic cell therapy is a promising therapy for breast cancer treatment.
Introduction
Cancer immunotherapy is a promising therapy for cancer treatment that uses the body’s immune system to fight cancer. There are several strategies that employ immune-cell based therapies in cancer treatment. The first strategy is to create a new immune system that can recognize and kill tumors after hematopoietic stem cell transplantation. In the second, immune cells such as dendritic cells (DCs) are used to activate effector cells (e.g. T cells) of the patient’s body to kill tumor cells. The third strategy employs transplanted T cells and NK (natural killer) cells or CIK cells (cytokineinduced killer) to find, identify and kill cancer cells Palucka and Banchereau, 2012.
DC-based immunotherapy is one approach that holds tremendous potential in the treatment of cancer. To date, several DC-based immunotherapeutic clinical trials have been reported Coosemans et al., 2013Schuler et al., 2014. The fundamental principle of this therapy is generation of an antigen-specific cytotoxic T lymphocyte (CTL) response by antigen primed DCs in the patient. Both tumor antigens (tumor associated antigen-TAA) and DCs play key roles in this process. DCs are known as professional antigenpresenting cells (APCs) that work efficiently and precisely within the body. DCs primed with a variety of effective TAAs have shown tumor response bothin vitro and vivo. There are many kinds of tumor-derived antigens commonly used in the treatment of cancer, included tumor antigen, lysates, apoptotic bodies, heat shock proteins, and peptides from TAA Alaniz et al., 2014Benencia, 2014Gao et al., 2014Lee et al., 2014Milano and Krishnadath, 2014.
Dendritic cells are antigen presenting cells that capture and process antigens in vivo, converting proteins to peptides that are presented on major histo-compatibility complex (MHC) molecules and recognized by T cells Steinman RM, 2007. They play a significant role in initiating the adaptive immune response to eliminate bacteria and other pathogens, as well as conditioning the body against autoimmune diseases, and aiding in tumor removal. As the main cell involved in initialization and promotion of the body’s immune system, these cells have become an interesting tool for immunotherapy in the treatment of disease, especially in cancer treatment. Various tissues have been used to promote the creation of the DC vaccines in clinical trials, including bone marrow cells, umbilical cord blood, and menstrual blood Koga et al., 2008Phuc et al., 2011. The ex vivo manipulation of these cells has two main advantages: first, it allows for the ability to control DC quality, including DC type and desired marker expression; and second, it allows for the ability to choose the method of reintroduction into the body, for example via injection into a vein or directly into the tumor or lymph nodes.
The cell culture techniques and the availability of cytokines have allowed a considerable amount of DCs to be manipulated ex vivo. Thus, DC-based cancer immunotherapy is used in clinical studies to stimulate a tumor-specific immune response and gain effects in some clinical trials Hobo et al., 2013Vik-Mo et al., 2013. This strategy includes priming DCs with tumor antigens such as peptides or proteins, DNA or RNA, or whole cancerous cells. Some other studies have shown that peptide-pulsed DCs enhanced peptide-specific cytotoxic T-lymphocytes (CTLs) in melanoma, renal cell carcinoma, breast carcinoma, and ovarian carcinoma in some clinical trials Bohnenkamp et al., 2004Nakai et al., 2009Nestle et al., 1998. However, peptide-pulsed DCs are limited because they are restricted to selected peptides and are only effective for those patients who express HLA. Moreover, some studies show that cancer patients relapsed after treatment with several peptides based on peptide-pulsed DCs. In contrast to using peptides, using tumor cell lysates to induce dendritic cells permits exposure of the entire range of tumor antigens, including non-specific TAAs and antigens with point mutations. Moreover, the epitope of HLA class I and II can be presented by DCs, and activated both CD8+ and CD4 T cells Gao et al., 2014Steinman RM, 2007. Limitations of the method using tumor antigen lysate are that it may cause autoimmune disease by DCs representing non tumor-antigens Sharma et al., 2013. Some problems about the effectiveness of the tumor cell lysate in stimulating DCs as well as activating an immune response still remain controversial. However, clinical trials show that patients in metastatic cancer stages have been treated with cancer cell antigens treated with freeze-thaw cycles or necrosis prior to DCs pulsing Chang et al., 2002Gitlitz et al., 2003.
Breast cancer is the most common type of cancer in women. The percentage of breast cancer cases has rapidly increased in both developed and developing countries in recent years. More importantly, the ratio of cases exhibiting anti-tumor drug resistance has strongly increased. Although some novel therapies based on chemicals, antibodies, surgery, or radiation have been developed to prolong survival, rapid modification of breast cancer cell properties enables tumors to overcome the effects of these therapies. With this in mind, DC therapy was used in this study to evaluate its effects in a murine model bearing breast tumors. To optimize the experimental conditions, 4T1 cells, a murine breast cancer cell line, were used along with allogeneic dendritic cells.
Materials – Methods
4T1 cell culture and tumor cell lysate preparation
The mouse breast cancer cell line, 4T1 was supplied by American Type Culture Collection (ATCC, USA). Cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), L-glutamine and penicillin/streptomycin (all from Sigma-Aldrich, St Louis, MO) at 37°C in 5% CO2.
Tumor cell lysates were prepared as described previously Kotera et al., 2001. Cells grown to 70-80% confluency were detached with 0.25% trypsin/EDTA and washed two times with D-PBS. Tumor cell lysates were generated from 5 × 10 6 tumor cells by 3 rapid freeze-thaw cycles in liquid nitrogen and a water bath at 37°C. The time of freeze-thaw cycles was set individually to 3, 5, or 7 min in liquid nitrogen and 7 min in the water bath, to select which protocol was the best with the highest number of necrotic cells. The tumor cell lysates were then spun at 700 rpm at 4°C for 10 min to remove cellular debris, and the supernatant was collected. Protein concentration in the lysates was detected by the Bradford method Ernst and Zor, 2010. The protein component of lysates was detected by SDS-PAGE and stained with Coomassie Brilliant Blue (Brunelle and Green, 2014). All lysates were stored at -80°C until used.
To detect necrotic cells, the cells were stained with both Annexin V and PI and analyzed by flow cytometry (according to manufacturer’s instructions, BD Biosciences). Briefly, 4T1 tumor cells (5 × 106 cells/ml) in different protocols were washed and re-suspended in binding buffer. Then, 5 μl of Annexin V conjugated with FITC, and 10 μl of propidium iodide were added to each cell suspension. After 10-min incubations in the dark at room temperature, cells were analyzed on a FACSCalibur (BD Bioscience) using Cell Quest software (BD Biosciences).
Dendritic cell culture
Dendritic cells were produced according to a previously described method Pham Van Phuc, 2011. Briefly, bone marrow- derived mononuclear cells (MNCs) were collected by flushing femurs and tibias with RPMI 1640 medium (Sigma- Aldrich, St Louis, MO). After centrifugation at 2,000 rpm for five minutes, the MNCs were re-suspended in complete RPMI 1640 medium supplemented with 10% FBS, 1% Lglutamine, 1% penicillin/streptomycin, 20 ng/ml murine recombinant GM-CSF (SantaCruz Biotechnology, Canada) and 20 ng/ml murine recombinant IL-4 (SantaCruz Biotechnology, Canada). After 3 days, 75% of the medium was removed, and fresh medium was added. On day 6, dendritic cell maturation was induced by supplementing the complete medium with 100 ng/mL, 1000 ng/mL, or 10000 ng/mL tumor cell lysate, and TNF-α (100 ng/mL) as a control. After three days, cells were harvested and used in the next experiments.
To evaluate the phenotype of mature dendritic cells, the differentiated cells were stained with specific surface markers CD14 (CD14-FITC), CD40 (CD40-FITC), CD80 (CD80- PERCP), CD83 (CD83-APC), and CD86 (CD86-PE) in staining buffer (PBS pH 7.4, 0.5% BSA, 0.02% azide). The stained cells were analyzed by BD FACSCalibur flow cytometer.
Animal treatment
Female mice (6-8 weeks old) were injected with 107 4T1 tumor cells (suspended in 100 μl cold PBS) into the mammary gland. On day 3, tumor-bearing mice were treated with intravenous (i.v.) injections of DCs pulsed with tumor cell lysates in a total volume of 100 μl PBS. These tumor-bearing mice were separated into four groups receiving different doses of DCs: (1) injected with 5 × 104 mature DCs; (2) injected with 105 mature DCs; (3) injected with 106 mature DCs; (4) no treatment control. Blood samples were collected from all animals on days 3, 7 and 13 of the study. The marker CD4 was used to analyze the immune response of mice in all experimental groups. Diameter of tumor sizes was followed every two days.
Statistical analysis
Each experiment was repeated three times. All statistical analysis was performed using the GraphPad Prism software (v 6.0). The data were presented as mean values ± standard deviation (SD). Statistical analysis was performed using ANOVA followed by least-significant difference (LSD). The result with p < 0.05 was considered to be statistically significant.
Results
Preparation of cancer cell lysates
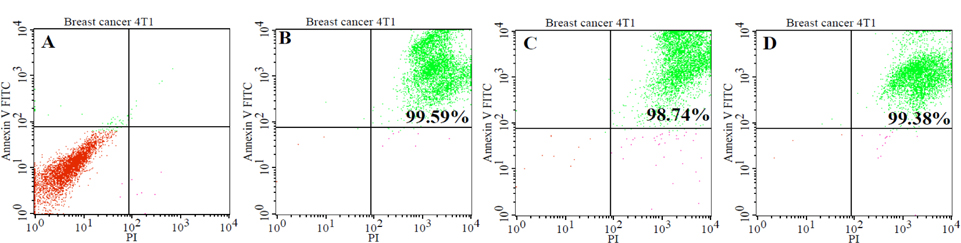
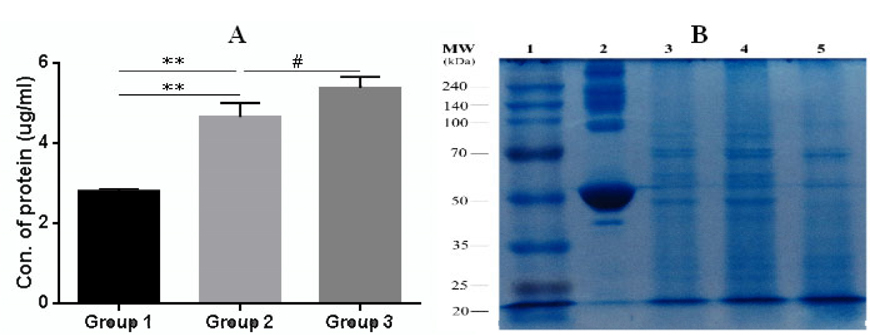
The rate of apoptosis and necrosis in tumor cells was evaluated by FACS analysis using a Annexin-V-FITC (Ann) and propidium iodide (PI) staining kit. FACS analysis for Annexin V-FITC and propidium iodide was able to differentiate between living cells (Ann V-PI-), cells in primary necrosis (Ann V-/PI+), cells in early apoptosis (Ann V+/PI-), and cells in secondary necrosis (Ann V+/PI+). The result of FACS analysis showed that most cells (98% ± 0.5; n = 3) were in secondary necrosis and this was true for all lysate samples ( Figure 1 ). For the quantification of protein concentrations, we used the Bradford method of protein quantification. The results showed the concentration of protein in all groups from 2.8 ± 0.06 μg/ml to 5.3 ± 0.2 μg/ml (p = 0.05; n = 3). The concentration of protein in group 3 was the highest, while the concentration of protein in group 1 was the lowest. To sum up, the lysate from the 4T1 cells which was broken in liquid nitrogen for three minutes before transfer to the water bath at 370C (group 1) had the lowest protein concentration, while the lysate from the 4T1 cells which was broken in liquid nitrogen for seven minutes then placed in the water bath at 37°C (group 3) had the highest protein concentration ( Figure 2A ).
To assess whether there were differences based on molecular weight of the proteins in the sample, SDS-PAGE was used for analysis ( Figure 2B ). Results showed that group 1 (lane 3) and group 2 (lane 4) had many fragments of protein with high molecular weight, while proteins in group 3 (lane 5) had lower molecular weight (<40 KDa). The low molecular weight protein fragments or peptides were suitable to induce maturation of dendritic cells Ahmadabad et al., 2011Hasan et al., 2012.
Dendritic cell differentiation
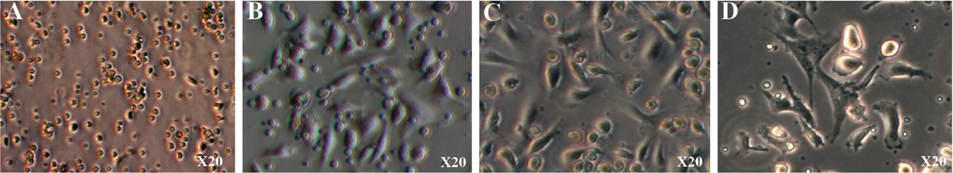
After 24 h of culture, the cells began to adhere to the surface of the culture flasks ( Figure 3A ). The cells spread on the surface of the culture flasks by the third day ( Figure 3B ). The next day, the monocytes transformed from round to stretched and adhered to the surface of the flasks. Some cells exhibited the particular shape of dendritic cells ( Figure 3C,D .
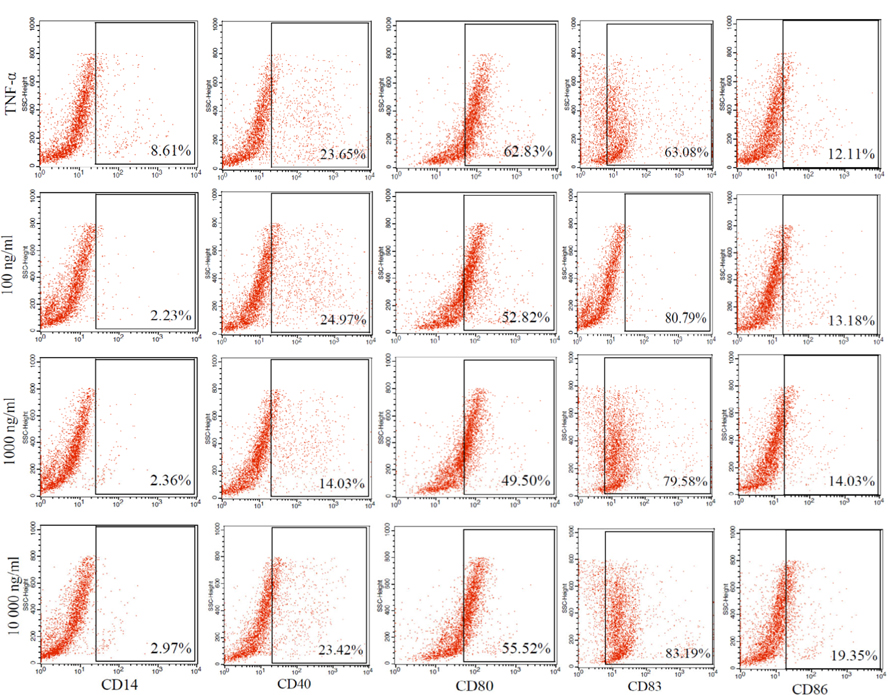
The maturation of tumor cell lysate-loaded DCs is shown in Figure 4 . Phenotypic analysis of dendritic cells showed that most of the cells were negative for CD14 marker in all groups. These cells expressed markers related to dendritic cells such as CD86 (12-19%), CD40 (14-24%), CD80 (49-62%), CD83 (63-83%) ( Figure 4 ). Statistical analysis showed no difference between the different antigen concentrations compared with the positive control group that matured by TNF-alpha. These results demonstrate that the mononuclear cells were induced to become mature dendritic cells.
Effects of tumor antigen-loaded DCs in the tumor-bearing mouse model
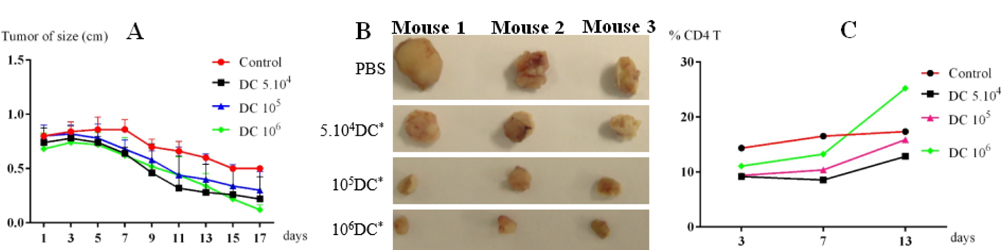
After three days, tumor-bearing mice were injected with tumor cell lysate-loaded DCs at different concentrations. Mice injected with PBS were used as controls. The mice immunized with tumor cell lysate-loaded DCs showed a significant improvement compared with the control group. After seven days, tumor growth in all groups injected with tumor cell lysate-loaded DCs was reduced in comparison with the control group. The reduction in growth of the tumor size did not differ between treatment groups from day 7 to day 13 (p = 0.05, n = 3). However, from day 15 to 17, these differences were significant between the group with 10 6 DCs injected compared with other groups (105 and 5 × 104 DCs injected) and the control group ( Figure 5A ). These results show that tumor-bearing mice had a positive response to injected lysate- pulsed DCs. Moreover, this response was the strongest at a dose of 10 6 DCs.
After 17 days immunization, tumors in all groups were obtained. The results showed that tumor sizes of treatment groups were significantly reduced versus the control. After 17 days, tumor size of the control group had decreased by 44%. Meanwhile, in the treated groups, tumor sizes were decreased to 43%, 50% and 87.5% of their original size respectively with the doses of injected DCs 5.104, 105, and 106 cells ( Figure 5B ). Tumor size in the experimental group injected with 106 tumor cells lysates-loaded DCs was more strongly decreased vs. the other groups.
To evaluate the immune response after DC injection, CD4+ cells percentages were counted and compared between different groups. The results were presented in Figure 5C . DCs trigger the immune system, especially T helper cells (CD4+ cells). CD4+ cell percentage gradually increased in a manner depending on the injected DC doses. In all investigated groups, CD4+ cell percentage strongly increased at dose of 106 DCs.
Discussion
Dendritic cells are professional antigen-presenting cells and play a central role in triggering an immune response. Therefore, dendritic cells are interesting for many studies. To this point, there have been many clinical studies of dendritic cells in cancer treatment that have shown promising results. By using a breast cancer model in mice and the breast cancer cell line 4T1 from mice, this study aimed to re-evaluate the effects of dendritic cell therapy in breast cancer treatment.
In this experiment, dendritic cells were successfully induced from bone marrow-derived mononuclear cells with tumor lysates and cytokines GMCSF and IL-4. These cells not only expressed the particular shapes of dendritic cells but also expressed dendritic cell-related markers such as CD40, CD80, CD86 and CD83. In fact, these proteins hold some important functions of DCs that help DCs to recognize and present the antigens to other immune cells, especially T cells and B cells. CD40 was initially characterized as a costimulatory molecule that played a central role in B and T cell activation Ma and Clark, 2009. CD80/CD86 costimulation on DCs is extremely essential during activation of naïve T cells into TH2 van Rijt et al., 2004. Moreover, CD83 acts as co-stimulatory signal for stimulation of naïve and memory T cells Aerts-Toegaert et al., 2007. To sum up, MNCs were successfully induced to become functional DCs, and these DCs were used in further experiments.
These DCs primed with tumor lysates exhibited their roles in vivo. In the next experiment, mature DCs injected into mice bearing tumors created by 4T1 cancer cells, efficiently reduced tumor growth. Moreover, effects of DC therapy had some particular characteristics. Firstly, the effect depended on the dose of DCs. At the low dose of DCs, 5 × 104 DCs almost did not have any effects to tumor growth. In fact, at this dose, the tumor size decreased to a similar extent as in the control. This difference was gradually significant when the dose of DCs increased to 105 and 106 cells/mouse. At a dose of 106 DCs, the tumor size was strongly reduced and exhibited half the size of the tumor in the control.
This study has been one of few studies that evaluate doses of DCs for treatment. In some previously published studies, doses of more than 106 DCs have been applied in DC therapy Gatza and Okada, 2002Kim et al., 2004Sato et al., 2003. At high dose of DCs, some previous authors also recorded that DC therapy gave many beneficial effects on breast cancer treatment in animal models as well as in humans, especially on prolonging survival. The first clinical trial using DCs in breast cancer treatment was performed in 2000 by Brossart et al. Brossart et al., 2000. In this study, DCs were derived from peripheral blood monocytes and induced by cytokines IL-4, GMCSF, and TNF-alpha for 7 days, and pulsed with peptides from Her-2/neu. The results showed that the vaccination of DCs could induce immunologic responses in patients and no significant side effects in patients Brossart et al., 2000. To date, there have been about 20 clinical trials carried out over the world involving breast cancer treatment using dendritic cells (according to clinicaltrial.gov). In recent years, some studies have improved the immune response by combining treatment with IL-12 injection Baek et al., 2011 or cytokine induced killer cells (Arafar, 2014).
The results also showed that effects of DC vaccination depended on the treating time. Significant differences concerning tumor size between treated groups and control were recognized after 14 days. This timing is consistent with the development of an immune response in the body. For further understanding, we analyzed the percentage of CD4 T cells in mice blood. At the high dose of DCs, after 14 days, CD4+ cells were strongly stimulated to expand and double the amount of DCs. This demonstrated that DCs triggered the immune system after being injected into peripheral blood. It is expected that DCs would migrate to the spleen after intravenous injection [10] or to lymph node [27] and then trigger a specific immune response. As antigen presenting cells, DCs would then activate the Th0 cells to become Th1 and Th2 cells Pal, 2014Thery and Amigorena, 2001. The high increase of CD4+ cells was evidence of stimulation by injected DCs. In other studies, DCs were also recognized as stimulators of CD8+ cells Pal, 2014Wang et al., 2014. Different to CD4+ cells that help activity of other immune cells by their released cytokines, CD8+ cells can kill cancer cells directly. By effecting CD4+ and CD8+ cells, DC vaccination could create a cancer cell-specific attack and eliminate these cells.
Conclusion
In summary, the finding of this study demonstrated that 4T1-cell derived antigen-primed DCs had the ability to inhibit breast tumor growth in mice. The highest efficacy of tumor reduction occurred at a dose of 106 DCs per mouse. Significant effects of DC vaccination were recorded after 14 days of treatment. DCs could efficiently stimulate to expand CD4+ cells. These results showed that DC therapy is a promising therapy for cancer treatment. This important role of DCs was recognized as an award to Ralph Steinman of the Nobel Prize for Medicine or Physiology in 2011. However, DC therapy for cancer might be a long road. Further experiments need to be performed in other models, especial breast tumors formed by breast cancer stem cells.
Abbreviations
DC: Dendritic cells; GMCSF: Granulocyte macrophage colony stimulating factor; IL-12: Interleukin 12; MNC: Mononuclear cells; TH: T helper cells; TNF-alpha: Tumor necrosis factor alpha
Authors’ contributions
All authors read and approved the final manuscript. VQP, TTM made the antigens from 4T1 cells; carried out the treatment experiments. STN and PKP prepared murine models; analyzed the data. PVP prepared the dendritic cells, prepared the manuscript in cooperation with all other authors.
References
-
C.
Aerts-Toegaert,
C.
Heirman,
S.
Tuyaerts,
J.
Corthals,
J.L.
Aerts,
A.
Bonehill,
K.
Thielemans,
K.
Breckpot.
CD83 expression on dendritic cells and T cells: correlation with effective immune responses. European journal of immunology.
2007;
37
:
686-695
.
-
H.N.
Ahmadabad,
Z.M.
Hassan,
E.
Safari,
M.
Bozorgmehr,
T.
Ghazanfari,
S.M.
Moazzeni.
Evaluation of the immunomodulatory effect of the 14 kDa protein isolated from aged garlic extract on dendritic cells. Cellular immunology.
2011;
269
:
90-95
.
-
L.
Alaniz,
M.M.
Rizzo,
G.
Mazzolini.
Pulsing dendritic cells with whole tumor cell lysates. Methods in molecular biology.
2014;
(Clifton
:
NJ) 1139, 27-31
.
-
A.
Arafar.
Cytokine induced killer cell immunotherapy in cancer treatment: from bench to bed side. Biomedical Research And Therapy.
2014;
1
:
71-77
.
-
S.
Baek,
C.S.
Kim,
S.B.
Kim,
Y.M.
Kim,
S.W.
Kwon,
Y.
Kim,
H.
Kim,
H.
Lee.
Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: results from a phase I/II trial. Journal of translational medicine.
2011;
9
:
178
.
-
F.
Benencia.
Antigen-specific mRNA transfection of autologous dendritic cells. Methods in molecular biology.
2014;
(Clifton
:
NJ) 1139, 77-86
.
-
H.R.
Bohnenkamp,
J.
Coleman,
J.M.
Burchell,
J.
Taylor- Papadimitriou,
T.
Noll.
Breast carcinoma cell lysate- pulsed dendritic cells cross-prime MUC1-specific CD8+ T cells identified by peptide-MHC-class-I tetramers. Cellular immunology.
2004;
231
:
112-125
.
-
P.
Brossart,
S.
Wirths,
G.
Stuhler,
V.L.
Reichardt,
L.
Kanz,
W.
Brugger.
Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood.
2000;
96
:
3102-3108
.
-
J.L.
Brunelle,
R.
Green.
One-dimensional SDS- Polyacrylamide Gel Electrophoresis (1D SDS-PAGE). Methods in enzymology.
2014;
541
:
151-159
.
-
A.E.
Chang,
B.G.
Redman,
J.R.
Whitfield,
B.J.
Nickoloff,
T.M.
Braun,
P.P.
Lee,
J.D.
Geiger,
J.J.
Mule.
A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clinical cancer research: an official journal of the American Association for Cancer Research.
2002;
8
:
1021-1032
.
-
A.
Coosemans,
A.
Vanderstraeten,
S.
Tuyaerts,
T.
Verschuere,
P.
Moerman,
Z.N.
Berneman,
I.
Vergote,
F.
Amant,
V.A.N.G.
SW.
Wilms’ Tumor Gene 1 (WT1)-loaded dendritic cell immunotherapy in patients with uterine tumors: a phase I/II clinical trial. Anticancer research.
2013;
33
:
5495-5500
.
-
O.
Ernst,
T.
Zor.
Linearization of the bradford protein assay. Journal of visualized experiments: JoVE 10.3791/1918.
2010
.
-
D.
Gao,
C.
Li,
X.
Xie,
P.
Zhao,
X.
Wei,
W.
Sun,
H.C.
Liu,
A.T.
Alexandrou,
J.
Jones,
R.
Zhao.
Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PloS one.
2014;
9
:
e93886
.
-
E.
Gatza,
C.Y.
Okada.
Tumor cell lysate-pulsed dendritic cells are more effective than TCR Id protein vaccines for active immunotherapy of T cell lymphoma. Journal of immunology.
2002;
(Baltimore
:
Md: 1950) 169, 5227-5235
.
-
B.J.
Gitlitz,
A.S.
Belldegrun,
A.
Zisman,
D.H.
Chao,
A.J.
Pantuck,
A.
Hinkel,
P.
Mulders,
N.
Moldawer,
C.L.
Tso,
R.A.
Figlin.
A pilot trial of tumor lysate-loaded dendritic cells for the treatment of metastatic renal cell carcinoma. Journal of immunotherapy.
2003;
(Hagerstown
:
Md: 1997) 26, 412-419
.
-
N.A.
Hasan,
M.H.
Zuhair,
E.
Safari,
M.
Bozorgmehr,
S.M.
Moazzeni.
Evaluation of the Effect of the 47 kDa Protein Isolated from Aged Garlic Extract on Dendritic Cells. Iranian journal of basic medical sciences.
2012;
15
:
745-751
.
-
W.
Hobo,
L.
Strobbe,
F.
Maas,
H.
Fredrix,
A.
Greupink-Draaisma,
B.
Esendam,
T.
de Witte,
F.
Preijers,
H.
Levenga,
B.
van Rees.
Immunogenicity of dendritic cells pulsed with MAGE3, Survivin and B-cell maturation antigen mRNA for vaccination of multiple myeloma patients. Cancer.
2013;
immunology
:
immunotherapy: CII 62, 1381-1392
.
-
K.W.
Kim,
S.H.
Kim,
J.G.
Shin,
G.S.
Kim,
Y.O.
Son,
S.W.
Park,
B.H.
Kwon,
D.W.
Kim,
C.H.
Lee,
M.Y.
Sol.
Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. International journal of cancer Journal international du cancer.
2004;
109
:
685-690
.
-
Y.
Koga,
A.
Matsuzaki,
A.
Suminoe,
H.
Hattori,
T.
Hara.
Expression of cytokine-associated genes in dendritic cells (DCs): comparison between adult peripheral blood- and umbilical cord blood-derived DCs by cDNA microarray. Immunology letters.
2008;
116
:
55-63
.
-
Y.
Kotera,
K.
Shimizu,
J.J.
Mule.
Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer research.
2001;
61
:
8105-8109
.
-
H.J.
Lee,
N.R.
Choi,
M.C.
Vo,
M.D.
Hoang,
Y.K.
Lee,
J.J.
Lee.
Generation of multiple Peptide cocktail-pulsed dendritic cells as a cancer vaccine. Methods in molecular biology.
2014;
(Clifton
:
NJ) 1139, 17-26
.
-
D.Y.
Ma,
E.A.
Clark.
The role of CD40 and CD154/CD40 L in dendritic cells. Seminars in immunology.
2009;
21
:
265-272
.
-
F.
Milano,
K.K.
Krishnadath.
Electroporation of dendritic cells with autologous total RNA from tumor material. Methods in molecular biology.
2014;
(Clifton
:
NJ) 1139, 87-95
.
-
N.
Nakai,
N.
Katoh,
W.T.
Germeraad,
T.
Kishida,
E.
Ueda,
H.
Takenaka,
O.
Mazda,
S.
Kishimoto.
Immunohistological analysis of peptide-induced delayed-type hypersensitivity in advanced melanoma patients treated with melanoma antigen-pulsed mature monocyte-derived dendritic cell vaccination. Journal of dermatological science.
2009;
53
:
40-47
.
-
F.O.
Nestle,
S.
Alijagic,
M.
Gilliet,
Y.
Sun,
S.
Grabbe,
R.
Dummer,
G.
Burg,
D.
Schadendorf.
Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature medicine.
1998;
4
:
328-332
.
-
C.
Pal.
Antigen trapping by dendritic cells for antitumor therapy. Methods in molecular biology.
2014;
(Clifton
:
NJ) 1139, 33-40
.
-
K.
Palucka,
J.
Banchereau.
Cancer immunotherapy via dendritic cells. Nature reviews Cancer.
2012;
12
:
265-277
.
-
C.J.H.
Pham Van Phuc,
Duong Thanh Thuy
Nguyen Thi Minh Nguyet.
Effects of breast cancer stem cell extract primed dendritic cell transplantation on breast cancer tumor murine models. Annual Review and Research in Biology.
2011;
1
:
13
.
-
P.V.
Phuc,
D.H.
Lam,
V.B.
Ngoc,
D.T.
Thu,
N.T.
Nguyet,
P.K.
Ngoc.
Production of functional dendritic cells from menstrual blood- a new dendritic cell source for immune therapy. In vitro cellular & developmental biology Animal.
2011;
47
:
368-375
.
-
M.
Sato,
K.
Chamoto,
T.
Nishimura.
A novel tumorvaccine cell therapy using bone marrow-derived dendritic cell type 1 and antigen-specific Th1 cells. International immunology.
2003;
15
:
837843
.
-
P.J.
Schuler,
M.
Harasymczuk,
C.
Visus,
A.
Deleo,
S.
Trivedi,
Y.
Lei,
A.
Argiris,
W.
Gooding,
L.H.
Butterfield,
T.L.
Whiteside.
Phase I Dendritic Cell p53 Peptide Vaccine for Head and Neck Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 10.1158/1078-0432.CCR-13-2617.
2014
.
-
R.K.
Sharma,
E.S.
Yolcu,
A.K.
Srivastava,
H.
Shirwan.
CD4+ T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4-1BBL and TAA-based vaccines in mouse tumor models. PloS one.
2013;
8
:
e73145
.
-
B.J.
Steinman RM.
Taking dendritic cells into medicine. Nature.
2007;
449
:
419-426
.
-
C.
Thery,
S.
Amigorena.
The cell biology of antigen presentation in dendritic cells. Current opinion in immunology.
2001;
13
:
4551
.
-
L.S.
Rijt,
N.
Vos,
M.
Willart,
A.
Kleinjan,
A.J.
Coyle,
H.C.
Hoogsteden,
B.N.
Lambrecht.
Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of TH2 effector responses in a mouse model of asthma. The Journal of allergy and clinical immunology.
2004;
114
:
166-173
.
-
E.O.
Vik-Mo,
M.
Nyakas,
B.V.
Mikkelsen,
M.C.
Moe,
P.
Due- Tonnesen,
E.M.
Suso,
S.
Saeboe-Larssen,
C.
Sandberg,
J.E.
Brinchmann,
E.
Helseth.
Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer, immunology and immunotherapy.
2013;
CII 62
:
1499-1509
.
-
Z.X.
Wang,
J.X.
Cao,
M.
Wang,
D.
Li,
Y.X.
Cui,
X.Y.
Zhang,
J.L.
Liu,
J.L.
Li.
Adoptive cellular immunotherapy for the treatment of patients with breast cancer: A meta-analysis. Cytotherapy.
2014;
16
:
934-945
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 03 (2014)
Page No.: 85-92
Published on: 2014-07-06
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8817 times
- Download PDF downloaded - 1745 times
- View Article downloaded - 13 times
 Biomedpress
Biomedpress