Abstract
Background: The risk factors of placenta previa differ around the world. This study evaluated risk factors of pregnancies complicated with placenta previa during a 5-year period in a referral center in Hamadan, Iran.
Methods: This case control study was conducted in Hamadan city (Hamadan Province of Iran) from April 2013 to March 2017. The cases were women whose deliveries were complicated by placenta previa and the controls were those who delivered without placenta previa. We recruited 130 cases and 130 controls. Multivariate unconditional logistic regression analysis was conducted, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
Results: The OR of placenta previa was 4.08 (95% CI= 1.44, 11.58) by maternal age, 4.08 (95% CI =1.44, 11.58) by preterm labor, and 6.64 (95% CI =1.09, 40.45) by prior operations of the uterine cavity, compared to normal deliveries and after adjusting for other variables. Multiparity, prior spontaneous abortions, and prior cesarean sections were not statistically significant risk factors for placenta previa, when adjusted for other variables.
Conclusion: Our study suggests that high maternal age and prior operations of the uterine cavity are risk factors for placenta previa.
Introduction
Placenta previa occurs when the placenta becomes implanted in the lower uterine segment or near the internal cervical os (opening into the uterus from the cervix). It takes place in less than 0.5% of all pregnancies. Placenta previa is correlated with morbidity and mortality of both mother and neonate Saleh Gargari et al., 2016. Placenta previa is created by invasion of placental villi beyond the decidua basalis which causes the placenta accreta or increta to form Miller et al., 1997.
Based on recent studies, several factors have been reported to contribute to placenta previa, including cesarean sections, smoking, abortions, assisted reproductive techniques (ART), and high-aged pregnancies (i.e. women of ages 35-45) Gibbins et al., 2017Karami et al., 2017Rasmussen et al., 2000Shobeiri and Jenabi, 2017Usta et al., 2005.
In 2003 a study in Croatia found that the risk factors for placenta previa to be: advanced age of mother (over 34 years of age), three or more previous pregnancies, parity of 2 and higher, high number of previous abortions, and history of previous cesarean sections Tuzovic et al., 2003. Additionally, a study in Austria in 2016 showed that risk factors and maternal outcomes were not related to the classification of placenta previa (major and minor placenta previa) Kollmann et al., 2016. Moreover, Kashani et al. in the north of Iran showed that placenta previa is clearly associated with prior cesarean sections Kashani et al., 2011.
Overall, the risk factor factors of placenta previa differ around the world Tuzovic et al., 2003 and studies of this topic have been limited in general. To our knowledge, no reports in this field have been published from the west side of Iran. In this study, we aimed to evaluate determinates of pregnancies complicated with placenta previa in Iran.
Materials-Methods
This case-control study was conducted in women who experienced childbirth complicated with placenta previa. Controls included women who had childbirth without complication at the Women's Hospital (Fatemieh, Hamadan City), Hamadan University of Medical Sciences, located in Western Iran from April 7 2013 to March 2017. This study was approved by the Student Research Committee of Hamadan University of Medical Sciences.
The inclusion criteria for the cases were:
(1) single pregnancy,
(2) no physical or mental diseases, and
(3) confirmation of placenta previa by sonographic imaging.
The exclusion criteria was women that had pregnancy failures or complications, according to hospital data record. Women in this study only had single pregnancies since twin pregnancies may intensify the effect of the risk factors Ananth et al., 2003.
Diagnosis of placenta previa was identified by transabdominal ultra sonographic imaging as conducted by the physician. The control subjects were matched to the cases by childbirth delivery method and by area of residence (rural or urban) since other variables could be considered as risk factors.
The sample size was based on results of a study conducted by Sohrabi et al. Sohrabi et al.. In the study, the proportion of control exposure was 49%, the proportion of cases with exposure was 78.5%; a two-sided type I error of 5 percent and 80 percent statistical power estimated a minimum of 41 for each group of case and control. For our study, during the 5-year period, 130 cases and 130 controls were selected. We used data records from the Fatemieh Hospital. Data were collected by a checklist, which included data on maternal age, parity, the area of residence, preterm labor (defined as a gestational age of less than 37 completed weeks), prior operations on the uterine cavity, prior spontaneous abortion, prior cesarean section, and prior ART. The validity and reliability of the checklist were assessed.
The logistic regression analysis was conducted to control the effect of various risk factors on placenta previa. Crude and adjusted odd ratios (ORS) were calculated to determine the association between placenta previa and risk factors by applying a significance level of 0.05 using the SPSS Statistics Software (V16.0, IBM Analytics Software, Chicago, IL).
Results
During the study period, 130 cases of placenta previa were confirmed. The mean (and standard deviation) of the maternal age of case and control groups was 28.90(±6.24) and 25.03(±5.97), respectively (p <0.1).
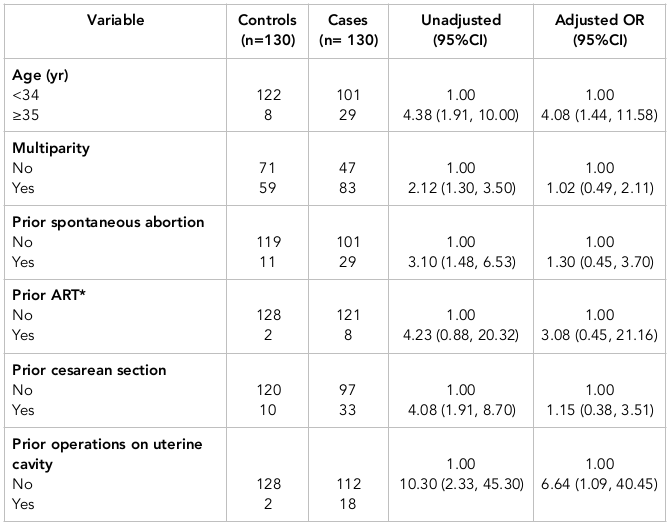
The characteristics of cases and controls are shown in Table 1 . Additionally, Table 2 shows the results of simple and multiple logistic regression analyses of the predictors of placenta previa. Adjustments (in multiple logistic regression analysis) were made for the following variables: age, parity, prior spontaneous abortions, prior ART, prior cesarean sections, gestational age, and prior operations on the uterine cavity.
There was a direct association between placenta previa and maternal age of 35 years or older. The OR estimate for placenta previa was 4.38 (95% CI=1.91, 10.00) in women aged 35 or older; this OR was higher in comparison to women less than 34 years of age. The OR estimates for placenta previa in women of 35 or older was 4.08 (95% CI=1.44, 11.58), when adjusted for other variables.
Moreover, there was a direct association between prior operations on the uterine cavity and placenta previa. The OR estimate of placenta previa in those with prior operations on the uterine cavity was 10.30 (95% CI =2.33, 45.30). When adjusted for other variables, the OR estimate was 6.64 (95% CI =1.09, 40.45).
Multiparity, prior spontaneous abortions, and prior cesarean sections all showed a statistically significant association with the risk of placenta previa. However, when adjusted for other variables, such an association did not persist. Indeed, prior ART was not significantly associated with placenta previa ( Table 2 ).
Discussion
The results of our study indicate that high maternal age and prior operations on the uterine cavity are risk factors for placenta previa. Several earlier studies Hung et al., 2007Saleh Gargari et al., 2016Tuzovic et al., 2003 have, indeed, shown that matrernal age and prior operations on uterine cavity can be risk factors for placenta previa. However, preterm delivery can result from placenta previa rather than being a risk factor for placenta previa.
Researchers have also conducted population-based case-control studies to assess the risk of placenta previa from prior cesarean section as opposed to prior vaginal delivery Kashani et al., 2011Rasmussen et al., 2000Tuzović et al., 2003, Yu et al., 2016. Rasmussen and Tuzovic reported that the risk of placenta previa was 1.32 and 2.0 fold higher after cesarean section than after vaginal delivery, respectively. According to the crude and adjusted OR estimates in our study, the risk of placenta previa was 4.08 and 1.15 in women who had prior cesarean section versus those who had prior vaginal delivery, respectively. Although the associations in multiple analyses were not found to be statistically significant, one possible reason for that could be low sample size.
Studies from Kollmann et al. Kollmann et al., 2016 and Tuzovic et al. Tuzovic et al., 2003 reported that women aged 35 or older, and with pariety of 2 or more, showed an increased the risk of placenta previa. In our study, the crude OR estimates were in line with previous studies which found advanced maternal age and high parity to be associated with an increased rate of placenta previa Hung et al., 2007Saleh Gargari et al., 2016Tuzovic et al., 2003Usta et al., 2005. Although these associations were not statistically significant, if the number of these events had been greater, the associations might be statistically significant. Another study conducted in Iran by Sohrabi et. al reported that prior cesarean, parity, age of the mother, prior abortions and prior placenta previa significantly increased the risk of placenta previa Sohrabi et al..
Based on a meta-analysis in 2016 Shobeiri and Jenabi, 2017, smoking is a key risk factor for placenta previa. In fact, smoking increased the risk of placenta previa by more than 1.2 fold. However, since women in case and control groups in our study did not smoke cigarrete, we could not estimate the OR associated with smoking.
The main limitation of our retrospective study is how to accurately assess the effect of certain risk factors on the outcome. To do so would require reliable sources of data, which is limited in our study. The quality and accuracy of the results depend primary on the quality of the recorded data; however, we were unable to verify the accuracy of the data which might result in data bias.
Conclusion
Our study reports that maternal age and prior operations on the uterine cavity are associeted with a risk of placenta previa. However, studies based on larger cohort and under different conditions are needed to fully validate our results. Awareness and education of determinates of placenta previa in pregnant women by midwives and obstetricians in health centers have the potential to reduce the risk of placentia previa during pregnancy.
Abbreviations
ART: Assisted Reproductive Techniques
CI: Confidence Interval
OR: Odds Ratio
Author contribution
EJ, Fs and MK designed the study. SK and EJ processed the data. MK and EJ performed the statistical analysis. EJ, Fs and MK interpreted the results. EJ, Fs, MK and SK wrote the first draft. EJ, FS and MK revised the final draft. All authors read and approved the final manuscript
References
-
Gargari S
Saleh,
Z
Seify,
L
Haghighi,
Shariati M
Khoshnood,
M
Mirzamoradi.
Risk Factors and Consequent Outcomes of Placenta Previa: Report From a Referral Center. Acta medica Iranica.
;
2016;54(11)
:
713-7
.
-
DA
Miller,
JA
Chollet,
TM
Goodwin.
Clinical risk factors for placenta previa-placenta accreta. American journal of obstetrics and gynecology.
;
1997;177(1)
:
210-4
.
-
F
Shobeiri,
E
Jenabi.
Smoking and placenta previa: a meta-analysis. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet.
;
2017
:
1-6
.
-
S
Rasmussen,
S
Albrechtsen,
K
Dalaker.
Obstetric history and the risk of placenta previa. Acta obstetricia et gynecologica Scandinavica.
;
2000;79(6)
:
502-7
.
-
IM
Usta,
EM
Hobeika,
AA
Musa,
GE
Gabriel,
AH
Nassar.
Placenta previa-accreta: risk factors and complications. American journal of obstetrics and gynecology.
;
2005;193(3 Pt 2)
:
1045-9
.
-
KJ
Gibbins,
BD
Einerson,
MW
Varner,
RM
Silver.
Placenta Previa and Maternal Hemorrhagic Morbidity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet.
;
2017
:
1-17
.
-
M
Karami,
E
Jenabi,
B
Fereidooni.
The association of placenta previa and assisted reproductive techniques: a meta-analysis. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet.
;
2017
:
1-8
.
-
L
Tuzovic,
J
Djelmis,
M
Ilijic.
Obstetric risk factors associated with placenta previa development: case-control study. Croatian medical journal.
;
2003;44(6)
:
728-33
.
-
M
Kollmann,
J
Gaulhofer,
U
Lang,
P
Klaritsch.
Placenta praevia: incidence, risk factors and outcome. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet.
;
2016;29(9)
:
1395-8
.
-
E
Kashani,
A
Tabandeh,
E
Zare,
G
Roshandel.
Risk factors and outcomes of placenta previa in pregnant women. Journal of Gorgan University of Medical Sciences.
;
2011;12(4)
:
Pe46-Pe50
.
-
CV
Ananth,
K
Demissie,
JC
Smulian,
AM
Vintzileos.
Placenta previa in singleton and twin births in the United States, 1989 through 1998: a comparison of risk factor profiles and associated conditions. American journal of obstetrics and gynecology.
;
2003;188(1)
:
275-81
.
-
D
Sohrabi,
F
Assadi,
M
Shamseddin.
Preavalance and Rrisk Factors of Placenta Previa in Valie Asr Hospital of Zanjan. Scientific Journal of Hamadan Nursing & Midwifery Faculty.
;
15(1)
:
11-21
.
-
TH
Hung,
CC
Hsieh,
JJ
Hsu,
TH
Chiu,
LM
Lo,
TT
Hsieh.
Risk factors for placenta previa in an Asian population. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics.
;
2007;97(1):
:
26-30
.
-
L
Tuzović,
J
Djelmiš,
M
Ilijić.
Obstetric Risk Factors Associated with Placenta Previa Development: Case-Control Study. Croatian medical journal.
;
2003;44(6)
:
728-33
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 06 (2017)
Page No.: 1411-1419
Published on: 2017-06-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 13349 times
- Download PDF downloaded - 2513 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress