Abstract
Background: Diabetes mellitus (DM) represents one of the greatest threats to modern global health. DM may affect male reproductive function at multiple levels as a result of its effects on spermatogenesis, sperm motility, sperm morphology, and change in sperm structure.
Methods: The present study deals with sperm motility and sperm morphological changes associated with diabetes in the male population. In this study, 50 insulin-dependent and 50 metformin users were selected, with ages of males ranging from 26-54 years and duration of diabetes distributed over 3-15 years. Both insulin-dependent and metformin-using diabetic subjects were evaluated for sperm analysis.
Results: Sperm analysis data showed a significant increase (p ±0.0005) in total sperm count in insulin-dependent diabetic men. However, sperm motility was found to be about 10-15% less in insulin-dependent patients compared to metformin users. Moreover, sperm morphology was improved in 6% of metformin users compared to insulin-dependent diabetics.
Conclusion: Our study concludes that metformin does not significantly affect sperm count. However, it does significantly affect sperm motility, when compared to insulin-dependent diabetic men. This study established an important relationship between diabetes and sperm motility, which reflects the reproductive capabilities of men.
Introduction
Diabetes mellitus (DM) is a metabolic disorder that is generally characterized as Type 1 diabetes (T1D) or Type 2 diabetes (T2D). T1D arises from a complete lack of insulin due to defective insulin secretion. T2D arises from a partial lack of insulin and is associated with alterations in carbohydrate, protein and lipid metabolism Association, 2014. In fact, T2D can result from a combination of inadequate insulin secretion and insulin resistance Association, 2010. In T2D, the symptoms can be detected only when the disease is in its advanced phase. Indeed, the changes produced in tissues and at the cellular level may not be reversed even when treated with the appropriate drug therapy Donner and Munoz, 2012.
DM is one of the most prominent population health threats in developed societies, with incidence on the rise each year. In 2002, the World Health Organization (WHO) reported that there were about 171 million DM patients worldwide; that number represented a 60% higher incidence compared to data from 1995 Organization, 2002. DM has been closely linked to long-term complications related to mortality and morbidity, such as hypertension and kidney failure McBrien et al., 2012Torgerson et al., 2004.
Insulin stimulates glucose uptake in muscle and fat cells. In the muscles, glucose is taken up and stored as glycogen; in fat cells glucose is absorbed and converted mostly to glycerin for long-term storage. The primary role of insulin is glucose transport into the plasma membrane of muscle and fat cells. The change in glucose transport activity due to the exposure to insulin brought changes and therefore different enzymes may act on different type of carbohydrates and fat metabolism takes place Denton et al., 1981.
Metformin is an oral diabetes drug which helps control blood sugar levels, particularly in those with type 2 diabetes. Metformin primarily acts on hepatic glucose ejection by inhibiting gluconeogenesis. However, it can cause weight loss and affect adipose tissues Stumvoll et al., 1995. The metabolism and mechanism of action of metformin are still poorly understood, despite the fact that it has been a form of medicine (for non-insulin-dependent diabetes mellitus (NIDDM)) for the past for 30 years.
DM is responsible for various homeostatic and biochemical alterations resulting in male infertility of sub-infertility. Individuals with diabetes have described sexual problems such as weakened sexual desire, likely related to a hyperglycemic state Ewing, 1985. Another sexual disorder linked to diabetes is erectile dysfunction Sexton and Jarow, 1997 and a decline in ejaculation Fedele, 2005. There appears to be a direct relationship between the incidence of DM and fertility, and incidence of DM and decrease in birth rates Lutz, 2006. The prevalence of DM in patients of reproductive age is a purported cause. The majority of patients are diagnosed with T1D before the age of 30 years and the rest are diagnosed with T2D Agbaje et al., 2007Silink, 2002.
In DM the molecular mechanisms and specific pathways, which induce defects in the male reproductive system and its functions, are both impacted. Spermatogenesis is altered in DM patients Daubresse et al., 1978. Several research studies comparing young or adult diabetics (to control individuals) have shown that diabetic males have lower sperm counts and significant changes in sperm morphology and motility Baccetti et al., 2002. Indeed, DM induces change in sperm count as well as sperm volume Bartak et al., 1974. Some other studies have shown that the concentration and sperm count are increased in diabetics but that sperm motility and semen volume are decreased Ali et al., 1993.
Blood glucose concentrations depend upon and adjust the function of different tissues and organs. Liver and fat are normally known to have strict control on glucose variations and are particularly known to play key roles in the use and storage of nutrients by hormonally regulated mechanisms. In testes, glucose metabolism is a vital event. Spermatogenesis preservation in vivo relies on glucose metabolism despite the fact that there are low levels of this sugar in tubular liquid Robinson and Fritz, 1981Zysk et al., 1975. Metformin helps to restore the body's response to insulin, making it possible to produce the amount of glucose and gastrointestinal absorption needed from the liver. Metformin (a biguanide derivative) reduces complications associated with blood glucose control.
Some studies have reported that there is no affiliation between age, sperm motility, T1D diagnosis and span of illness. In the study herein, we demonstrate that the parameters used to assess sperm motility (e.g. progressive velocity, path velocity, way speed and lateral head displacement) remained unchanged. For example, the direct linear index (which unveils the straightness of sperm swimming) was expanded in diabetic patients and normal individuals Niven et al., 1995. Our study shows that the effect of T1D on male fertility may be associated to the complications of the disease and not to the disease itself. The sperm cells of diabetic males are reported to have high fructose and glucose content. However, a relationship could not be established between an ineffective metabolic control and the observed alterations in the semen. A broad study of spermatozoa cryopreservation from patients with diverse pathologies showed that only sperm from diabetic men presented considerable differences in the parameters used to assess sperm Ranganathan et al., 2002. Despite the fact that there is some ambiguity with regards to sperm parameters, there appears to be a genuine effect on the regenerative arrangement of males with DM. Furthermore, aside from the immediate studies on sperm, some new and critical results have been confirmed by in vitro hormonal tests. For example, diabetic patients do not show changes in sex hormones such as testosterone Ding et al., 2006.
Thus, hormonal control of sperm cell metabolism has a direct effect on spermatogenesis O'Donnell et al., 2017 and should deserve special attention when studying metabolic diseases that are also related to hormonal regulation or lack of. Moreover, individuals with diabetes have severe insulin deregulation, which continues to be an important consideration factor when studying DM. Euglycemia is very difficult to maintain in diabetic patients. Hyperinsulinemia / hypoglycemia and hypoglycemia / hyperinsulinemia are common events faced by diabetic patients daily. Thus, insulin can play a highly important role in male sexual dysfunction in those afflicted with DM. In fact, recent research reports show that only a few hours of insulin deficiency can alter sperm cell glucose metabolism Alves et al., 2013 and completely suppress in vitro acetate production Alves et al., 2012. The role of insulin in processes vital for normal spermatogenesis clearly indicates that the molecular mechanisms by which DM impacts male reproductive function may also be associated with insulin fluctuations and glucose concentrations.
Methods
In our comparative study, 100 subjects were evaluated. There were 50 participants with insulin-dependent diabetes mellitus (IDDM), with ages ranging from 26-54 years. Likewise, there were 50 participants with NIDDM, with ages ranging from 35-54 years. All diabetic subjects involved in the study were males.
Inclusion criteria included
- Male patients with T1D receiving insulin only
- Male patients with T2D receiving metformin only
Exclusion criteria included
- Patients with pelvic surgery
- Patients with hernia repair
- Patients with thyroid disease
- Patients with hypertension
- Patients with testicular cancer
- Patients on tamoxifen
- Patients with prostate cancer
- Patients with nephropathy
- Patients with neuropathy
- Patients with testicular varicocele
- Patients with genital infections
- Patients with leukocytospermia
- Patients with chronic illnesses
- Patients with serious systemic diseases
Methods
The collection and examination of semen were done by properly standardized procedures according to WHO guidelines. Semen samples were collected by masturbation after a recommended sexual abstinence period of 2–5 days. Spermatozoa for ICSI were prepared using density gradient method. During the research study, tests were conducted on semen volume, semen pH, semen count, semen morphology, sperm motility, and serum. Testosterone levels were conducted using computer-assisted semen analysis (CASA) according to WHO recommendation WHO, 1999 and DNA fragmentation analysis by SCD (define abbreviation). All data were analyzed using computer software. Analysis of variance (ANOVA) test was applied to compare the efficacy of groups. P-value < 0.05 was considered statistically significant.
Ethical Approval
The study was approved by the Research and Ethics Committee (REC) of Faculty of Pharmacy, University of Lahore, Lahore-Pakistan (Ethical-No (EN-3121-2016) dated 12-09-2016).
Results
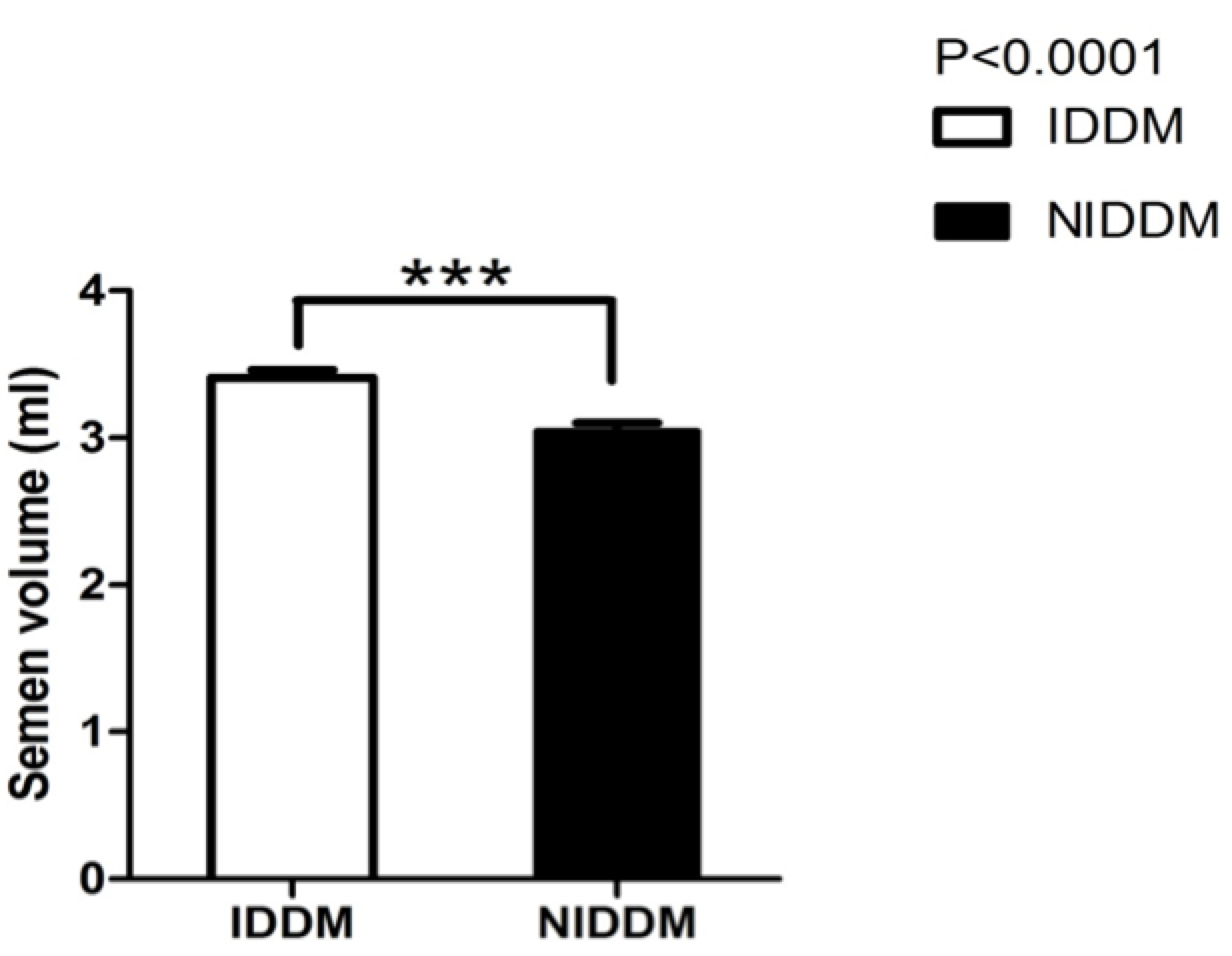
Only 100 male patients met the inclusion criteria. Each took the required tests which were used to assess sperm motility, sperm morphology, semen profile, and semen pH. Semen volume was also evaluated, and the effect of insulin and metformin on diabetic patients were also compared. Figure 1 describes the mean difference between semen volume of insulin and metformin users.
Discussion
DM represents one of the greatest threats to modern global health. DM may affect male reproductive functioning at multiple levels as a result of its effects on spermatogenesis, sperm motility, sperm morphology, and change in sperm structure. The present study evaluated sperm motility and sperm morphological changes associated with diabetes in males. In the study, metformin- and insulin-dependent diabetic subjects underwent multiple tests for sperm analysis. The results show that there was an increase in semen volume in patients receiving insulin (IDDM), when compared to those using metformin (NIDDM) ( Figure 1 ). Similar results have been reported by Bosman et al., who conducted a clinical study to investigate the effect of metformin and antioxidant treatment on semen parameters of hyper-insulinemia in men. They found that both groups significantly differed (p<0.05) in sperm morphology. The enhancement of sperm morphology was similar for both groups after treatment, and normal sperm morphology was increased for both groups. Thus, the Bosman et al. study showed that infertile hyperinsulinemic men can benefit from metformin treatment and should be advised to use nutritional supplements with antioxidant properties Bosman et al., 2014.
In another study, the appropriate treatment of oligo-terato-asthenozoospermic patients with metabolic syndrome was evaluated Morgante et al., 2011. In the study, 45 patients were treated with metformin for a duration of 6 months. The use of metformin was associated with a statistically significant reduction of insulin resistance and sex hormone–binding globulin levels. Moreover, a statistically significant increase in serum androgen levels and an improvement in semen characteristics were seen Morgante et al., 2011.
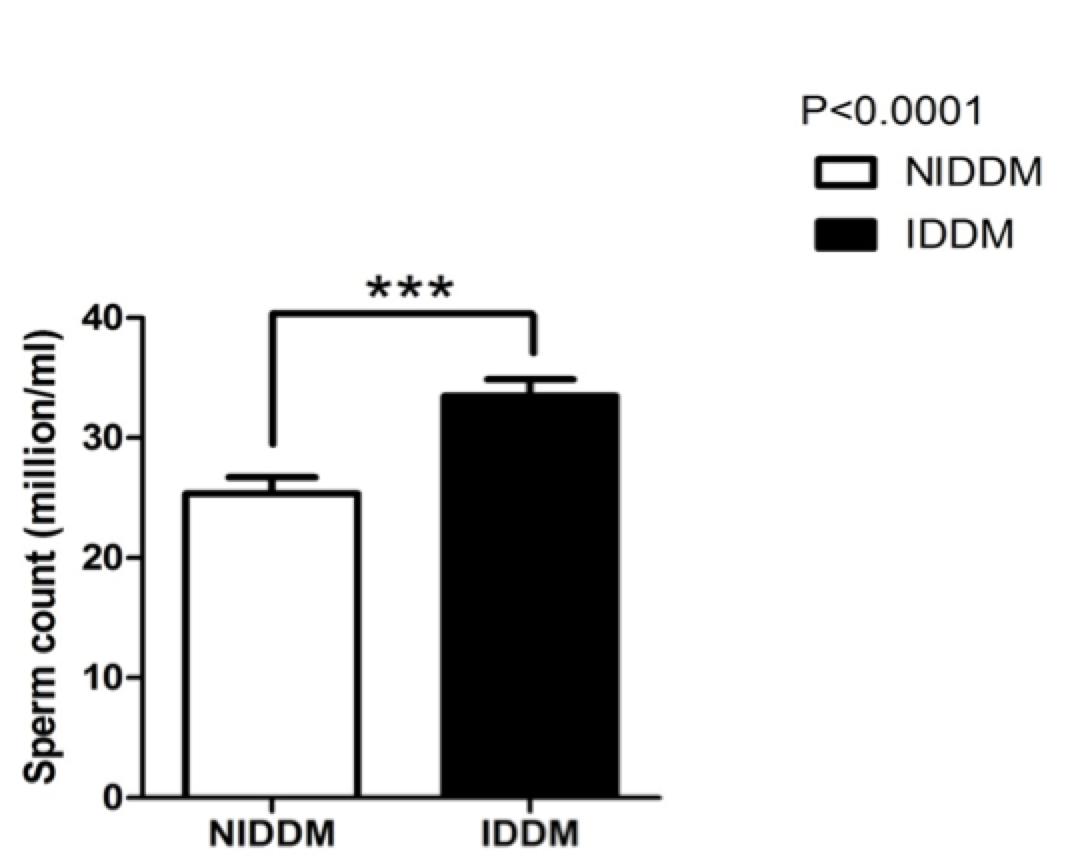
Insulin not only affects semen volume but also modifies sperm count ( Figure 2 ). Our study also confirmed this; we found that sperm count was significantly increased (p<0.0001) in insulin users compared to metformin users. Our study also showed that sperm morphology was not significantly changed by metformin use ( Figure 3 ). However, insulin use affected sperm morphology. Metformin users (NIDDM) had better sperm morphology (p<0.0001) as compared to insulin-dependent diabetics (IDDM).
Other research studies have examined the effects of dietary zinc depletion on seminal volume, serum testosterone concentration, and sperm morphology in young men Hunt et al., 1992. Identification of the andrological variables most sensitive to zinc depletion would expedite the diagnosis of male reproductive pathology induced by zinc deficiency. The findings from that study suggest that serum testosterone concentrations, seminal volume, and total seminal zinc loss per ejaculate are sensitive to short-term zinc depletion in young men Hunt et al., 1992.
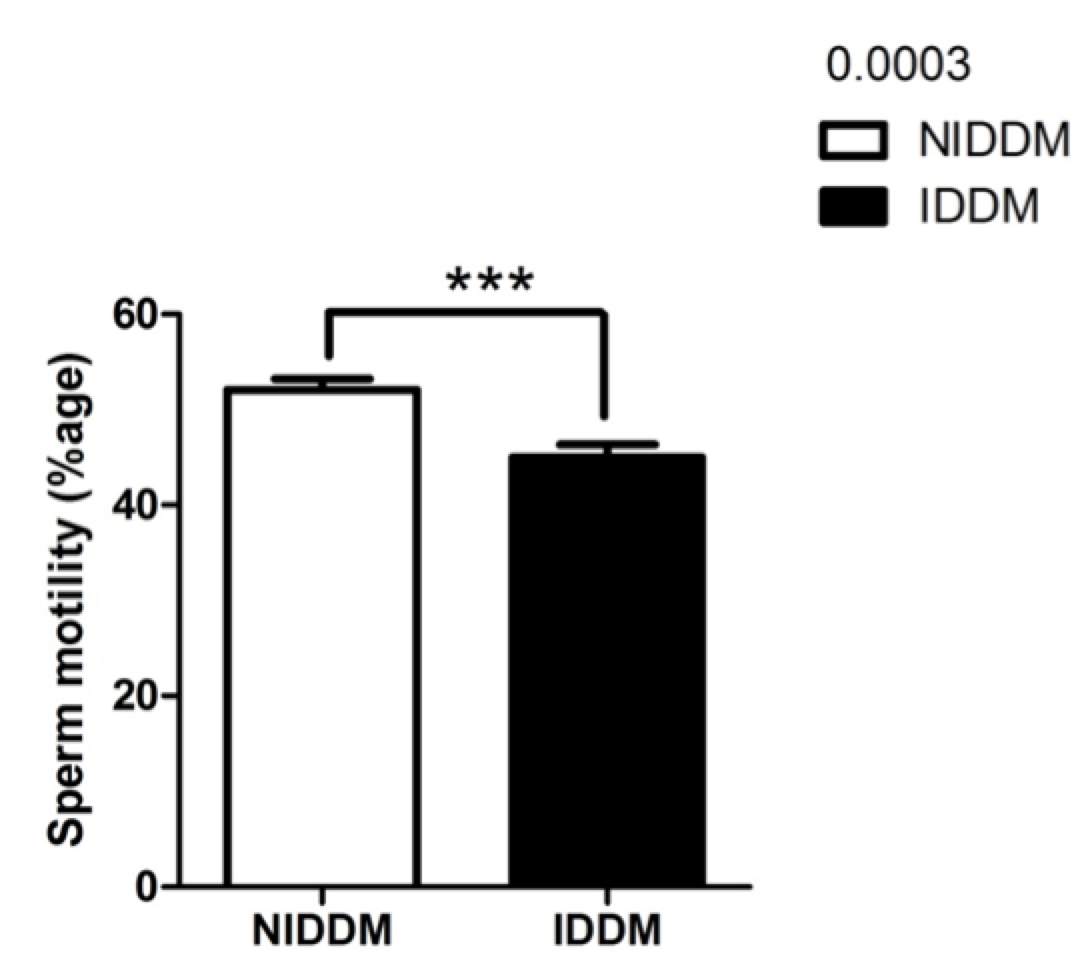
Sperm motility in our study was significantly improved (p<0.0003) in metformin users as compared to insulin-dependent diabetics ( Figure 4 ). Recently, a research study examined the effects of insulin-like growth factors (IGFs) and IGF-binding proteins (IGFBPs) on in vitro sperm motility Miao et al., 1998. The study concluded that IGF-I and IGFBP-3 have differing and opposing effects on in vitro sperm motility parameters and thus may different roles in modulating in vivo sperm motility Pitteloud et al., 2005a. Other studies have investigated the in vitro effects of insulin and leptin on human sperm motility, viability, acrosome reaction, and nitric oxide (NO) production Lampiao and Du Plessis, 2008. Their results showed that insulin and leptin significantly increased total motility,progressive motility and acrosome reaction, as well as NO production. Thus, there appears to be in vitro beneficial effects of insulin and leptin on human sperm function; these hormones could play a role in enhancing the fertilization capacity of human spermatozoa Lampiao and Du Plessis, 2008.
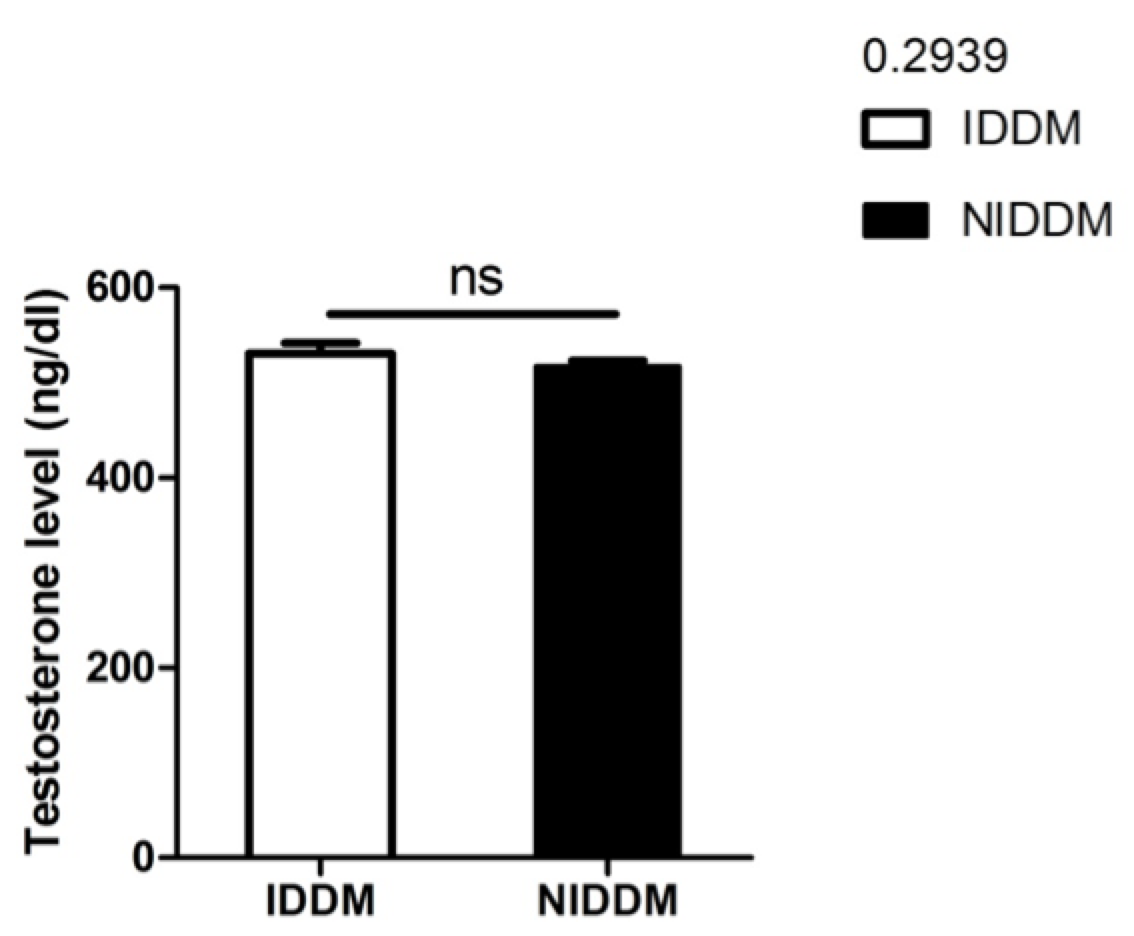
In our study, although metformin improved sperm motility, neither metformin nor insulin significantly affected serum testosterone levels (p<0.2939) ( Figure 5 ). However, the data from our study does establish a relationship between insulin and serum testosterone level. Pitteloud et al. (2005) previously examined the relationship of serum testosterone levels to insulin sensitivity and to mitochondrial function in men. Their results demonstrated that approximately 45% of subjects in their study had normal glucose tolerance, 20% had impaired glucose tolerance, and 35% had T2D. Testosterone levels were positively correlated with insulin sensitivity (r = 0.4, P < 0.005). Subjects with hypogonadal testosterone levels (n = 10) had a BMI >25 kg/m2 and a 3-fold higher prevalence of metabolic syndromes than their eugonadal counterparts (n = 50). This relationship held true after adjusting for age and sex hormone–binding globulin but not for BMI. Testosterone levels also correlated with Vo2max (r = 0.43, P<0.05) and oxidative phosphorylation gene expression (r = 0.57, P<0.0001). Their data indicated that low serum testosterone levels are associated with an adverse metabolic profile and suggest a novel unifying mechanism for previously independent observations that low testosterone levels and impaired mitochondrial function promote insulin resistance in men Pitteloud et al., 2005b.
Pitteloud et al. (2013) further investigated and found that increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. They evaluated the hypothalamic-pituitary-gonadal axis in men with a spectrum of insulin sensitivity. From their data, it was concluded that insulin resistance is, indeed, associated with a decrease in Leydig cell T secretion in men. However, additional studies are required to determine the mechanisms of the effect Pitteloud et al., 2005a.
Conclusion
From our present study, we conclude that insulin-dependent male diabetes have improved semen volume, sperm morphology and sperm count. On the other hand, sperm motility was better in metformin users than insulin users. However, both insulin and metformin use did not significantly affect serum testosterone levels in the diabetes. Therefore, we conclude that metformin might play a better role than insulin in enhancement of sperm motility in the diabetic male population.
Abbreviations
CASA: Computer-assisted semen analysis
DM: Diabetes Mellitus
IDDM: Insulin Dependent Diabetes Mellitus
NIDDM: Non-Insulin Dependent Diabetes Mellitus
SCD: Sperm Chromatin Dispersion
T1D: Type 1 Diabetes
T2D: Type 2 Diabetes
Author contribution
Awais Ali Zaidi and Arsalan Ali conducted the research, Mahtab Ahmad Khan, Lubna Shakir and Ali Sharif Supervised the study, Awais Ali Zaidi and Zaib Ali Shaheryar drafted the manuscript, Atif Irshad Reviewed the manuscript, Data analysis performed by Mahtab Ahmad Khan.
References
-
I.
Agbaje,
D.
Rogers,
C.
McVicar,
N.
McClure,
A.
Atkinson,
C.
Mallidis,
S.
Lewis.
Insulin dependant diabetes mellitus: implications for male reproductive function. Human Reproduction.
2007;
22
:
1871-1877
.
-
S.
Ali,
R.
Shaikh,
N.
Siddiqi,
P.
Siddiqi.
Semen analysis in insulin-dependent/non-insulin-dependent diabetic men with/without neuropathy. Systems Biology in Reproductive Medicine.
1993;
30
:
47-54
.
-
M.
Alves,
A.
Martins,
L.
Rato,
P.
Moreira,
S.
Socorro,
P.
Oliveira.
Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease.
2013;
1832
:
626-635
.
-
M.G.
Alves,
S.
Socorro,
J.
Silva,
A.
Barros,
M.
Sousa,
J.E.
Cavaco,
P.F.
Oliveira.
In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17β-estradiol and suppressed by insulin deprivation. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research.
2012;
1823
:
1389-1394
.
-
A.D.
Association.
Diagnosis and classification of diabetes mellitus. Diabetes Care.
2010;
33
:
S62-S69
.
-
A.D.
Association.
Diagnosis and classification of diabetes mellitus. Diabetes Care.
2014;
37
:
S81-S90
.
-
B.
Baccetti,
A.
la Marca,
P.
Piomboni,
S.
Capitani,
E.
Bruni,
F.
Petraglia,
V.
De Leo.
Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Human Reproduction.
2002;
17
:
2673-2677
.
-
V.
Bartak,
M.
Josifko,
M.
Horackova.
Juvenile diabetes and human sperm quality. International journal of fertility.
1974;
20
:
30-32
.
-
E.
Bosman,
A.
Esterhuizen,
F.
Rodrigues,
P.
Becker,
W.
Hoffmann.
Effect of metformin therapy and dietary supplements on semen parameters in hyperinsulinaemic males. Andrologia.
2014;
47
:
974-979
.
-
J.
Daubresse,
J.
Meunier,
J.
Wilmotte,
A.
Luyckx,
P.
Lefebvre.
Pituitary-testicular axis in diabetic men with and without sexual impotence. Diabete & metabolisme.
1978;
4
:
233-237
.
-
R.
Denton,
R.
Brownsey,
G.
Belsham.
A partial view of the mechanism of insulin action. Diabetologia.
1981;
21
:
347-362
.
-
E.L.
Ding,
Y.
Song,
V.S.
Malik,
S.
Liu.
Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. Jama.
2006;
295
:
1288-1299
.
-
T.
Donner,
M.
Munoz.
Update on insulin therapy for type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism.
2012;
97
:
1405-1413
.
-
D.
Ewing.
Sexual dysfunction in diabetic men. Practical Diabetes International.
1985;
2
:
6-9
.
-
D.
Fedele.
Therapy Insight: sexual and bladder dysfunction associated with diabetes mellitus. Nature Clinical Practice Urology.
2005;
2
:
282-290
.
-
C.D.
Hunt,
P.E.
Johnson,
J.
Herbel,
L.K.
Mullen.
Effects of dietary zinc depletion on seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. The American journal of clinical nutrition.
1992;
56
:
148-157
.
-
F.
Lampiao,
S.S.
Du Plessis.
Insulin and leptin enhance human sperm motility, acrosome reaction and nitric oxide production. Asian journal of andrology.
2008;
10
:
799-807
.
-
W.
Lutz.
Fertility rates and future population trends: will Europe's birth rate recover or continue to decline?. International Journal of Andrology.
2006;
29
:
25-33
.
-
K.
McBrien,
D.M.
Rabi,
N.
Campbell,
L.
Barnieh,
F.
Clement,
B.R.
Hemmelgarn,
M.
Tonelli,
L.A.
Leiter,
S.W.
Klarenbach,
B.J.
Manns.
Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Archives of internal medicine.
2012;
172
:
1296-1303
.
-
Z.R.
Miao,
T.K.
Lin,
T.
Bongso,
X.
Zhou,
P.
Cohen,
K.O.
Lee.
Effect of insulin‐like growth factors (IGFs) and IGF‐binding proteins on in vitro sperm motility. Clinical endocrinology.
1998;
49
:
235-239
.
-
G.
Morgante,
C.
Tosti,
R.
Orvieto,
M.C.
Musacchio,
P.
Piomboni,
V.
De Leo.
Metformin improves semen characteristics of oligo-terato -asthenozoospermic men with metabolic syndrome. Fertility and sterility.
2011;
95
:
2150-2152
.
-
M.
Niven,
G.
Hitman,
D.
Badenoch.
A study of spermatozoal motility in type 1 diabetes mellitus. Diabetic medicine.
1995;
12
:
921-924
.
-
L.
O'Donnell,
P.
Stanton,
D.M.
de Kretser.
Endocrinology of the Male Reproductive System and Spermatogenesis. In Endotext, C.G. De Groot LJ, Dungan K, et al., ed. (South Dartmouth (MA)).
2017
.
-
W.H.
Organization.
Diabetes: the cost of diabetes (Fact sheet No. 236). World health organization (WHO) www who intlmediacentre/factsheets/fs236/en.
2002
.
-
N.
Pitteloud,
M.
Hardin,
A.A.
Dwyer,
E.
Valassi,
M.
Yialamas,
D.
Elahi,
F.J.
Hayes.
Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. The Journal of Clinical Endocrinology & Metabolism.
2005a;
90
:
2636-2641
.
-
N.
Pitteloud,
V.K.
Mootha,
A.A.
Dwyer,
M.
Hardin,
H.
Lee,
K.-F.
Eriksson,
D.
Tripathy,
M.
Yialamas,
L.
Groop,
D.
Elahi.
Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care.
2005b;
28
:
1636-1642
.
-
P.
Ranganathan,
A.M.
Mahran,
J.
Hallak,
A.
Agarwal.
Sperm cryopreservation for men with nonmalignant, systemic diseases: a descriptive study. Journal of andrology.
2002;
23
:
71-75
.
-
R.
Robinson,
I.B.
Fritz.
Metabolism of glucose by Sertoli cells in culture. Biology of reproduction.
1981;
24
:
1032-1041
.
-
W.J.
Sexton,
J.P.
Jarow.
Effect of diabetes mellitus upon male reproductive function. Urology.
1997;
49
:
508-513
.
-
M.
Silink.
Childhood diabetes: a global perspective. Hormone Research in Paediatrics.
2002;
57
:
1-5
.
-
M.
Stumvoll,
N.
Nurjhan,
G.
Perriello,
G.
Dailey,
J.E.
Gerich.
Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. New England Journal of Medicine.
1995;
333
:
550-554
.
-
J.S.
Torgerson,
J.
Hauptman,
M.N.
Boldrin,
L.
Sjöström.
XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care.
2004;
27
:
155-161
.
-
WHO.
WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge university press.
1999
.
-
J.
Zysk,
A.
Bushway,
R.
Whistler,
W.
Carlton.
Temporary sterility produced in male mice by 5-thio-D-glucose. Journal of reproduction and fertility.
1975;
45
:
69-72
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 06 (2017)
Page No.: 1388-1399
Published on: 2017-06-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8970 times
- Download PDF downloaded - 2113 times
- View Article downloaded - 15 times
 Biomedpress
Biomedpress