Abstract
Background: The application of mesenchymal stem cell (MSC) therapy in liver fibrosis treatment has been increasingly investigated in recent years. MSCs obtained from a variety of sources (e.g. bone marrow, umbilical cord blood and adipose tissue) have been studied and have achieved remarkable results. In this study, we compared the effects of adipose-derived mesenchymal stem cells (AD-MSC) transplantation with bone marrow-derived mesenchymal stem cell (BM-MSC) transplantation in a mouse model of liver fibrosis, induced by carbon tetrachloride (CCl4).
Methods: Eight-week old mice were treated with CCl4 for 11 weeks to induce liver fibrosis then 5x105 cells were transplanted into mice via the tail vein.
Results: After 21 days of transplantation, the results showed that the stem cell treated groups ameliorated better than the placebo group. MSC treated groups showed reduced AST and ALT levels, down-regulated expression of extracellular matrix (ECM) genes, and improved liver histopathology. Both sources of MSCs (bone marrow and adipose tissue) were effective in the mouse model of liver fibrosis.
Conclusion: Our results also indicated that AD-MSC transplantation in mice accelerated liver regeneration better than BM-MSC transplantation.
Introduction
Liver cirrhosis is a serious disease with a high mortality risk, ranking among the top ten causes of death in Eastern Europe, Central Asia and high-income countries Mortality and Causes of Death, 2015. The number of patients with liver cirrhosis worldwide in 2015 was estimated at more than 39 million; this number represents an increase of approximately 2 million, compared to that of 2005 Kassebaum et al., 2016. Common causes of liver cirrhosis include hepatitis B virus (HBV), hepatitis C virus (HCV), and alcohol abuse. By histology, the hallmark features of cirrhosis are diffuse nodules in the liver. The fibrotic tissues are formed by excessive accumulation of the extracellular matrix. Liver cirrhosis leads to severe consequences such as portal hypertension, ascites, liver failure and death. To date, liver transplantation has been the gold standard choice for patients with advanced cirrhosis. However, this therapy is high-risk due to graft rejection, viral re-infection, and complications of long-term immunosuppressive treatment Schuppan and Afdhal, 2008.
MSCs have many prominent properties which are related to their capability of liver tissue regeneration; these include differentiation into hepatocyte-like cell in vitro Harn et al., 2012, immune modulation Bifari et al., 2008, reduction of activation of hepatic stellate cells Baligar et al., 2016, and amelioration of hepatitis Seki et al., 2013. In recent years, many studies have been conducted using MSCs for the treatment of cirrhosis. The sources of MSCs are diverse, such as cord blood Abdel Aziz et al., 2010Choudhery et al., 2013, bone marrow Abdel Aziz et al., 2007Yuan et al., 2014Zhao et al., 2005, and adipose tissue Choudhery et al., 2013Seki et al., 2013Zhang et al., 2014. Although these studies have shown that MSCs are a promising therapeutic choice for liver fibrosis Berardis et al., 2015, the origin of MSCs from different sources may be problematic, with the varying safety and effectiveness of the cells Reinisch et al., 2015. Therefore, more robust investigations on the varied sources of MSCs are necessary for approving MSC application for therapy. In this study, we evaluated the effectiveness of MSCs obtained from two sources (bone marrow and adipose tissue) to study how MSCs from different sources influence the therapeutic efficacy.
Materials - Methods
Cirrhosis model mouse
Eight-week-old male Swiss mice were used in this study. The mice were treated with 1.0 mL/kg (99.5% purity, UNI-CHEM Chemical Reagent, China) 3 times per week for 11 weeks. Prior to CCl4 administration, mice were fasted for 4 h. The animal study was accepted by the Institutional Ethics Committee of SCL Laboratories (Laboratory of Stem Cell Research and Application, University of Science, VNU-HCM, Vietnam).
AD-MSC isolation
Adipose tissue was isolated from mouse testicles and stored in PBS containing 10% antibiotic- antimycotic, 100 X (Gibco/Thermal Fisher Scientific, Waltham, MA). Tissues were washed 2 times with PBS containing 10% antibiotic-antimycotic. The ADSC extraction kit (Geneworld Ltd. Co, Saigon High-tech Park, Ho Chi Minh, Vietnam) was used according to the manufacturer’s instructions to separate out stromal vascular fractions (SVFs). The SVF component (containing cells) was then cultured in a 25 cm2 Roux flask containing complete DMEM/F-12 medium (i.e. DMEM/F-12 medium (Sigma-Aldrich, St. Louis, MO), 15% fetal bovine serum (FBS) (Gibco, Waltham, MA), and 1% of 100X antibiotic-antimycotic (Gibco). The cells were cultured at 37°C, 5% CO2. The medium was changed every 3 days.
BM-MSC isolation
The femurs of 4-week old male mice were collected and stored in PBS containing 10% of 100X antibiotic-antimycotic (Gibco) for bone marrow extraction. Muscle, tendon and cartilage were removed. Mononuclear cells (BM-MNCs) were isolated from bone marrow by flushing with medium through a 22-G needle to obtain single cells. BM-MNCs were cultured in a 25 cm2 flask in complete DMEM/F-12 medium at 37°C, 5% CO2. The medium was changed every 3 days.
Characterization of AD-MSCs and BM-MSCs
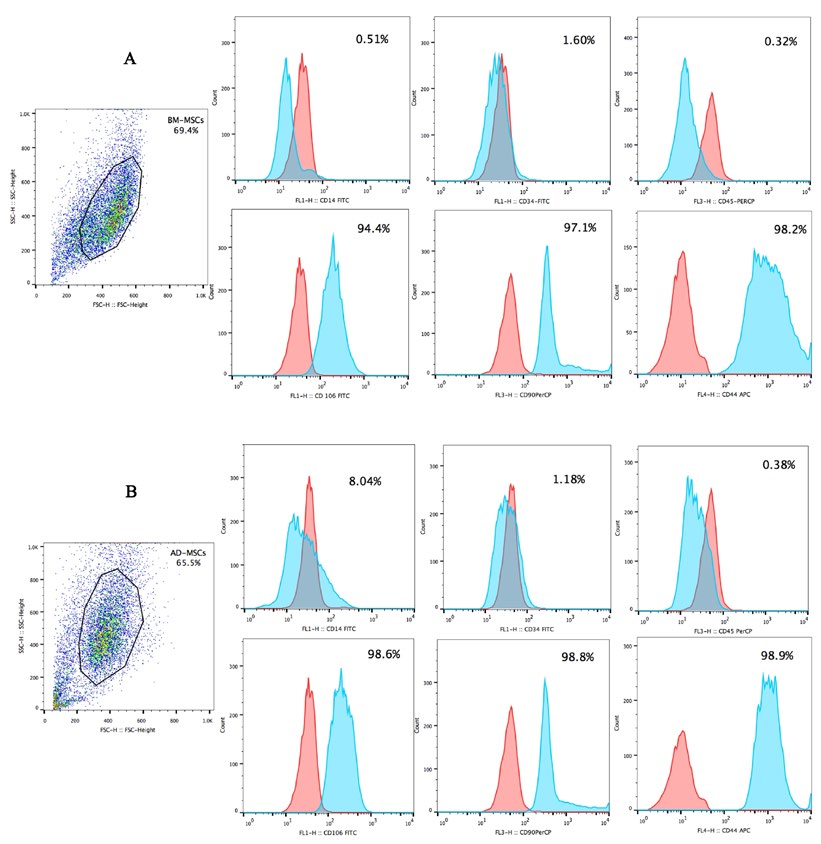
After the 3rd passage, candidate cells were evaluated for MSCs markers and differentiation capacity into adipogenic cells. In brief, MSCs markers were assessed by flow cytometry on a FACSCalibur system (BD Biosciences, Franklin Lakes, NJ) using FITC-, PerCP-, PE- or APC-conjugated anti-CD34, anti-CD45, anti-CD14, anti-CD44, anti-CD90, and anti-CD106 antibodies.
In this study, we tested the adipogenic potential of MSCs using adipogenic induction medium (i.e. DMEM/F-12 supplemented with 10% FBS, 1% of 100X antibiotic-antimycotic solution, 10mM dexamethasone (Sigma-Aldrich), 2.79 mM indomethacin (Sigma-Aldrich), 5 mg/mL insulin (Sigma-Aldrich), and 0.5 M 1-Methyl-3-isobutylxanthine (IBMX) (Sigma-Aldrich). After 21 days of differentiation, the differentiated cells were assessed by oil red staining.
AD-MSC and BM-MSC transplantation
Mice with liver fibrosis were divided into 3 groups (n=5 per group). The groups were as follows: Group 1 (Placebo; PBS injection), Group 2 (AD-MSC treatment), and Group 3 (BM-MSC treatment). Passage 3 (P3) MSCs were trypsinized with 0.25% trypsin and washed with PBS. MSCs were filtered through a 70 μm filter to obtain single cells then 5x105 MSCs were resuspended in 0.15 ml PBS and injected into the tail vein.
Evaluations after AD-MSC and BM-MSC transplantation
After 21 days of transplantation, mice were evaluated for:
Level of AST, ALT and ALB in serum
200 μL of plasma from each mouse was collected to assess the level of AST (IFCC Mod.LiquiUV test, Germany), ALT (IFCC Mod. LiquiUV test, Germany) and ALB (QuantiChrom Bilirubin Assay Kit, Bioassay Systems, CA, USA), according to the manufacturer’s instructions.
Expression of fibrosis related genes
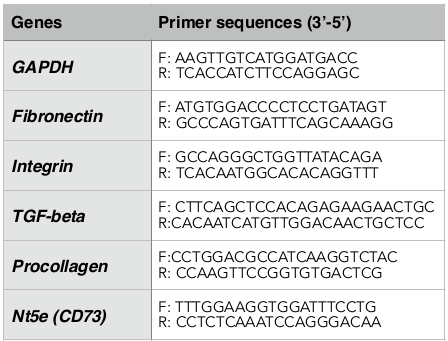
Liver tissues in the left liver lobe were obtained for RNA extraction using the Easy_BLUE Total RNA Extraction Kit (INTRON Biotechnology, Korea) according to the manufacturer’s instructions. Gene expression was determined by quantitative RT-PCR method (Brilliant II QRT-PCR Master Mix Kit 1-Step, Agilent, CA) using the specific primers listed in Table 1 .
Histopathology
Liver tissues (approximately 1 cm2) were obtained from left liver lobes. The samples were washed with PBS two times and fixed in paraformaldehyde for 24 h before sectioning in paraffin. For hematoxylin & eosin (H&E) staining, liver sections were deparaffinized in xylene, dehydrated using alcohol, and washed with PBS. Liver sections was then stained in hematoxylin (Merck Millipore, Germany) for 5 min, washed quickly, and then differentiated by 1% acid alcohol for 30 seconds then washed for 10 min. Slides were stained with eosin solution (Merck Millipore, Germany) for 2-3 min, washed and then mounted. For Masson staining, liver sections were deparaffinized, dehydrated and washed. Slides were stained with Weigert’s iron hematoxylin for 5 min, washed, then stained with Biebrich scarlet acid fuchsin solution for 5 min and washed again. Slides were differentiated in 1% phosphomolybdic-phosphotungstic acid solution for 5 min, then transferred to aniline blue solution, and stained for 5 min. Finally, sections were differentiated in 1% acetic acid solution for 1 min, washed, dehydrated, and mounted with mounting medium.
Statistical Analysis
Data analysis was conducted using GraphPad Prism 6 software (La Jolla, CA). Statistical significance was set at p<0.05.
Results
Isolating and characterizing MSCs
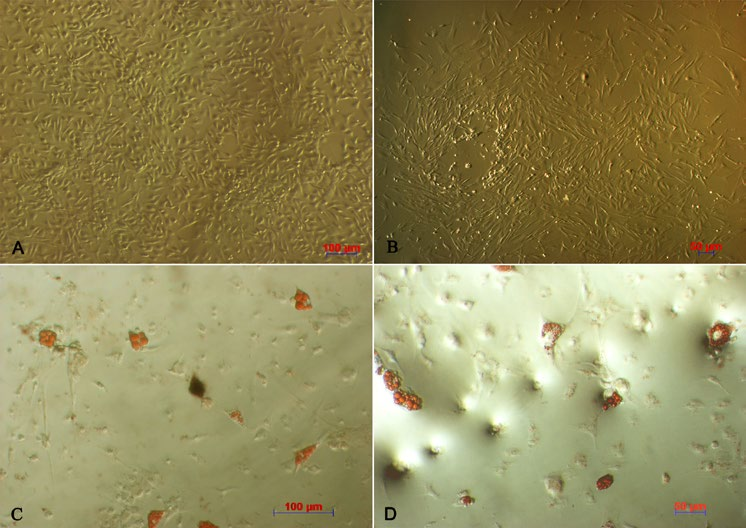
After culturing primary cells for 48 h, a number of cells adhered and spread onto the surface; these cells had a spindle shape. At the third passage, candidate cells showed the distinct fibroblast-like morphology ( Figure 1A,B ).
When induced by differentiation media into adipogenic cells, the morphology of the MSCs changed into a round shape and lipid droplets accumulated in the cytoplasm. The globular shape was caused by gradual enlargement of the droplets, pushing nuclei aside ( Figure 1C,D ). The P3 candidate cells derived from adipose tissue and bone marrow were positive for CD44, CD90, and CD106 expression, but negative for CD14, CD34, and CD45 expression ( Figure 2 ).
Changes in Liver Injury/Function Markers after MSC transplantation
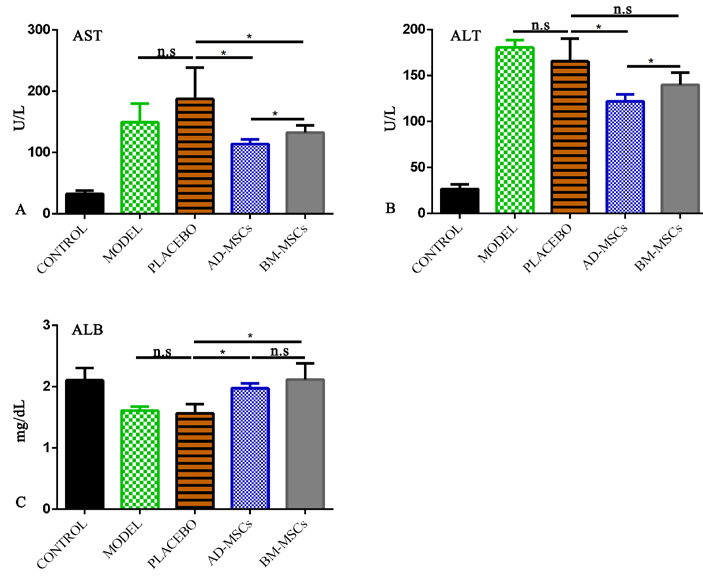
Administration of either AD-MSCs or BM-MSCs improved liver injury and liver function as compared to those injected with PBS. Levels of AST (114.2±3.2 U/L) and ALT (122.6±3.5 U/L) in the AD-MSC group were significantly lower than in the BM-MSC group ( Figure 3A,B ). Moreover, MSC-treated groups showed an improvement in ALB index compared to the placebo group. However, there were no significant differences between the two MSC-treated groups ( Figure 3C ).
Lower expression of ECM-related genes in groups administered with MSCs
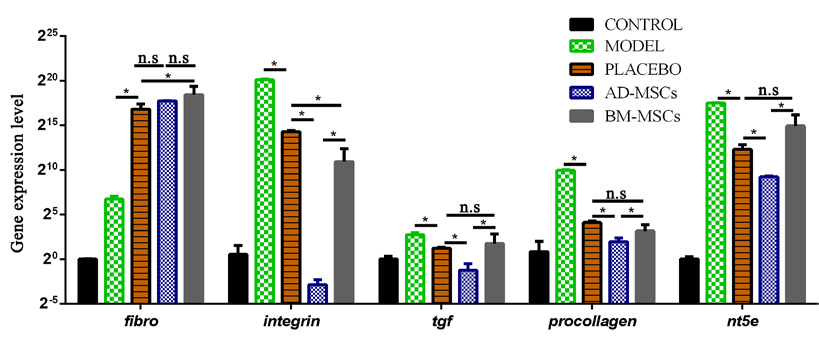
An upregulation of the fibronectin gene but downregulation of the other ECM-related genes were observed in all groups administered with PBS or stem cells (p<0.05) after 21 days of treatment ( Figure 4 ). However, the expression of fibrosis-associated genes was significantly lower in the AD-MSC treated group than in the placebo group (p<0.05). In the BM-MSC treated group, there was an observed decline in the expression of integrin, compared to the placebo group; none of the other genes showed significant differences in their expression (p>0.05).
Histological analyses of groups treated with stem cells show evidence of reconstruction
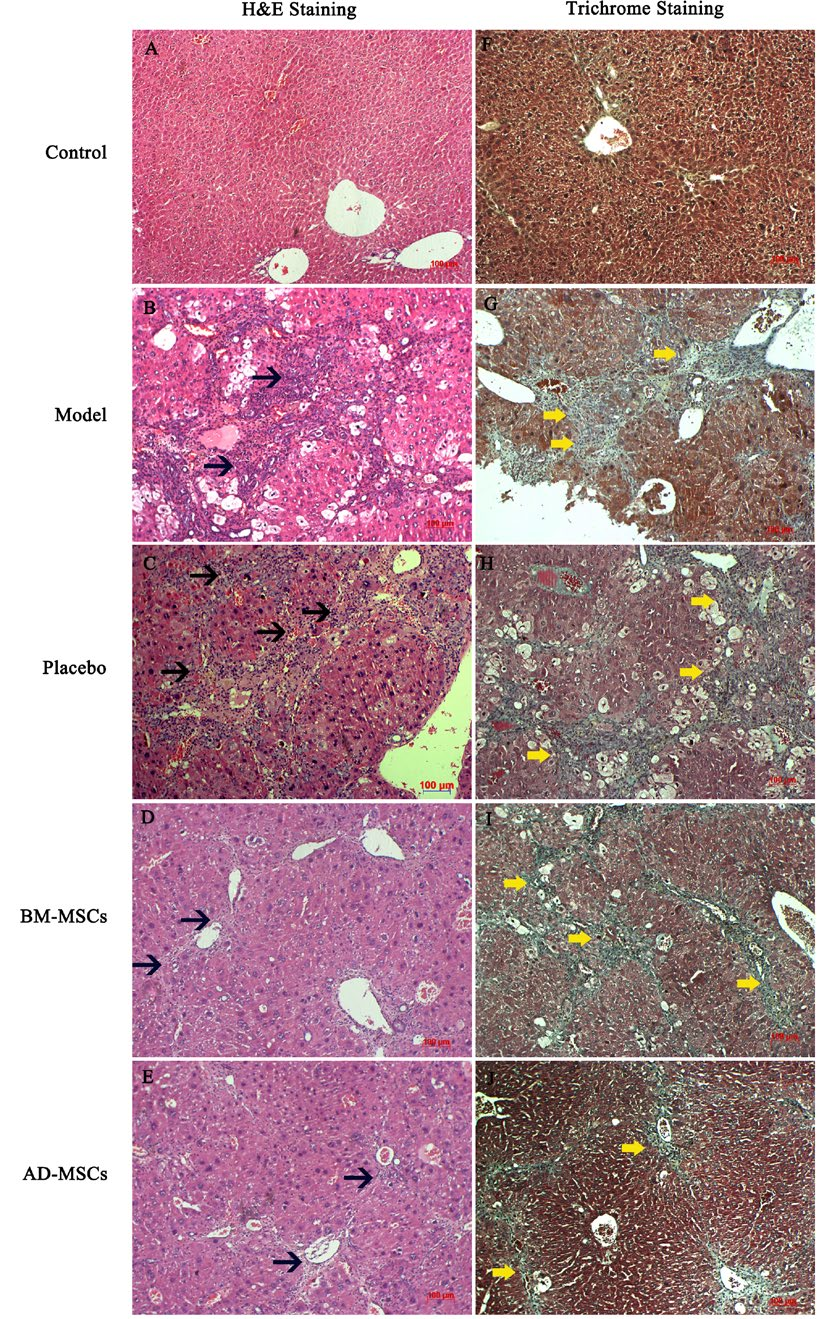
In PBS-treated mice, apoptotic cells were detectable and persistent inflammation led to a dense distribution of immune cells in close proximity to the portal vein, central vein and lobes. As well, degenerative swelling of liver cells was observed ( Figure 5C ). Collagen fibers were distributed extensively among the liver tissues and fibrosis bridges were seen in the portal-central, portal-portal areas, as revealed by Masson’s trichrome staining ( Figure 5H ). On the other hand, the inflammatory condition improved in the stem cell treated groups ( Figure 5D,E ). Correspondingly, degeneration of liver cells was relieved and collagen fibers ceased to accumulate as densely or widely in the liver. There was no fibrosis bridge observed in the MSC treated groups ( Figure 5I,J ).
Discussion
In this study, we did not use hepatotoxin during the MSC transplantation process. During treatment, it is important to eliminate pathogens so as to efficiently treat the disease without introducing unnecessary complications. Such a strategy has the advantage of boosting the patient's therapy regimen. However, a major disadvantage is the self-recovery and regeneration of the mouse liver due to CCl4 ceasing. Previous studies using mouse models of liver cirrhosis have indicated that mice livers are affected and fibrotic conditions improve after CCl4 is withdrawn Mederacke, 2013. This explains the recovery of AST/ALT and the expression of the fibrosis gene in the placebo group. However, the groups injected with MSCs all showed improvement (which was statistically significant) when compared to the PBS-treated group. These results suggest that administration of MSCs can improve injured livers. In treatment, a faster, more effective recovery is beneficial. The treatment efficiency of MSCs can occur due to secretion of immunomodulatory factors and low expression of MHC (MHCII expression is virtually absent) Koppula et al., 2009Nicolay et al., 2015. As mentioned above, elevation of AST and ALT levels as well as decline of albumin concentration, account for hepatocyte damage.
In this study, the improvement of the biochemical indexes observed in both groups transplanted with MSCs suggests a protective effect of MSCs on hepatocytes. The protective effect might be explained by the ability of MSCs to secrete cytokines such as hepatocyte growth factor (HGF) Matsuda-Hashii et al., 2004 and nerve growth factor (NGF). As well, MSCs can secrete anti-inflammatory molecules, such as interleukin (IL)-10 and interleukin 1 receptor antagonist (IL1-RA), which have anti-apoptotic and proliferative effects on hepatocytes and which help improve liver functions Francois et al., 2013. Other studies have revealed the role of MSC transplantation in improving oxidative stress in cells within damaged areas Eirin et al., 2015. The alleviation of liver fibrosis in MSC-treated groups could be related to the paracrine mechanism of MSCs and their differentiation into functional cells; paracrine mechanisms are the predominant means by which MSCs exert their action Berardis et al., 2015Parekkadan et al., 2007.
Co-culturing of MSCs and hepatic stellate cells (HSCs) can lead to an increase in the number of G0-stage HSCs, which is consistent with the decrease in number of S-stage HSCs. Such observations suggest a role for MSCs in inhibiting HSCs Zhao et al., 2005. Moreover, MSCs play a role in inhibiting the activation and proliferation of HSCs, thereby enhancing the clearance of abnormal ECM in fibrotic structures Parekkadan et al., 2007, and in the regulation of fibrosis-related genes Ali and Masoud, 2012. The modulatory effects of MSCs, as mentioned above, can be used to explain the results of the decline of expression of fibrosis-related genes in the treated groups, compared to placebo, in our study. However, one limitation in our study is that the mechanism of action of MSCs (e.g. paracrine secretion, differentiation into hepatocyte in vivo, and anti-fibrosis effects) has not been determined in our model. Further investigations into the mechanism of action of MSCs in CCl4- induced mouse model of liver fibrosis will aid in a greater understanding of MSCs in therapy.
Histological studies were consistent with gene expression results, suggesting an improvement in all of the groups when CCl4 administration was ceased. However, the alleviation in fibrosis occurred significantly faster in mice treated with MSCs than in untreated mice, reflecting the important role of MSCs. There are two mechanisms that could be associated with the clearance of ECM in fibrotic liver tissues: decrease of structural ECM proteins (mainly collagen) and elevation of enzymes (which dissolve the ECM). Research by Abdel et al. (2007) have indicated that MSC transplantation reduces collagen expression Abdel Aziz et al., 2007, that MSCs have an ability to inhibit activities of HSCs, and that MMPs secretion can result in decomposing the abnormal ECM in fibrotic livers.
In our study, there were various questions concerning AD-MSC and BM-MSC therapies, mainly whether or not the different types of stem cells from various sources would result in differences in treatment efficiency. Previous in vitro studies have proven that MSCs from various sources share a similarity in the differentiation potential or possibilities for therapeutic applications Choudhery et al., 2013. However, recent results from the study by Reinisch et al. (2015) have revealed that epigenetic or microenvironment factors in origin of stem cells significantly affect the potential of MSCs. Both in vitro and in vivo studies have suggested differences in differentiation capacity and genetic profile amongst populations of cells, regardless of similarities in cell morphology, surface markers or differentiation potential Reinisch et al., 2015. Studies by Bigot et al. (2015) have also indicated that the oxygen levels can affect the stability of the genetic profiles of AD-MSCs and BM-MSCs in different ways Bigot et al., 2015. These phenomena might account for variations in the efficiency of MSCs acquired from various sources for therapeutic applications.
Conclusion
All mice in the experiment groups showed improvement of their liver fibrosis; even mice in the placebo group showed improvement when CCl4 administration was ceased. Importantly, the MSC-treated group showed a great improvement in their AST/ALT ratios. Even histological structures in the treated mice looked significantly better than that of placebo. Overall, comparison of the efficiencies between the two sources of MSCs (AD-MSCs versus BM-MSCs), the AST/ALT ratios, and the gene expression and histology indicated that AD-MSCs were better as a therapeutic platform than BM-MSCs.
Abbreviations
AD-MSCs: Adipose tissue-derived mesenchymal stem cells
ALB: Albumin
ALT: Alanine transaminase
AST: Aspartate aminotransferase
BM-MNCs: Bone marrow-derived mononuclear cells
CCl4: Carbon tetrachloride
ECM: Extracellular matrix
HBV: Hepatitis B virus
HCV: Hepatitis C virus
HGF: Hepatocyte growth factor
HSC: Hepatic stellate cells
IL: Interleukin
SVFs: Stromal vascular fractions
VNU-HCM: Vietnam National University, Ho Chi Minh City
Author contribution
Nam Hai Nguyen carried out experiments, acquisition of data and compose the first manuscript. Trinh Van Le and Huy Quang Do conducted the mouse model, acquisition data of liver function, gene-expression. Huy Minh Le and Dat Quoc Ngo made substantial contributions to analyze the histology change. Nhung Hai Truong made substantial contributions to conception and design, data analysis and interpretation of data. Being corresponding author, Truong Hai Nhung give final approval of the manuscript to be submitted and any revised version.
References
-
M.T.
Abdel Aziz,
H.M.
Atta,
S.
Mahfouz,
H.H.
Fouad,
N.K.
Roshdy,
H.H.
Ahmed,
L.A.
Rashed,
D.
Sabry,
A.A.
Hassouna,
N.M.
Hasan.
Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clinical biochemistry.
2007;
40
:
893-899
.
-
M.T.
Abdel Aziz,
M.F.
El Asmar,
S.
Mostafa,
H.
Salama,
H.M.
Atta,
S.
Mahfouz,
N.K.
Roshdy,
L.A.
Rashed,
D.
Sabry,
N.
Hasan.
Reversal of Hepatic Fibrosis by Human CD34(+) Stem/Progenitor Cell Transplantation in Rats. International Journal of Stem Cells.
2010;
3
:
161-174
.
-
G.
Ali,
M.S.
Masoud.
Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl(4)-induced fibrotic liver. Saudi journal of gastroenterology : official journal of the Saudi Gastroenterology Association.
2012;
18
:
263-267
.
-
P.
Baligar,
S.
Mukherjee,
V.
Kochat,
A.
Rastogi,
A.
Mukhopadhyay.
Molecular and Cellular Functions Distinguish Superior Therapeutic Efficiency of Bone Marrow CD45 Cells Over Mesenchymal Stem Cells in Liver Cirrhosis. Stem cells.
2016;
34
:
135-147
.
-
S.
Berardis,
P.
Dwisthi Sattwika,
M.
Najimi,
E.M.
Sokal.
Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World journal of gastroenterology.
2015;
21
:
742-758
.
-
F.
Bifari,
V.
Lisi,
E.
Mimiola,
A.
Pasini,
M.
Krampera.
Immune Modulation by Mesenchymal Stem Cells. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie.
2008;
35
:
194-204
.
-
N.
Bigot,
A.
Mouche,
M.
Preti,
S.
Loisel,
M.L.
Renoud,
R.
Le Guevel,
L.
Sensebe,
K.
Tarte,
R.
Pedeux.
Hypoxia Differentially Modulates the Genomic Stability of Clinical-Grade ADSCs and BM-MSCs in Long-Term Culture. Stem cells.
2015;
33
:
3608-3620
.
-
M.S.
Choudhery,
M.
Badowski,
A.
Muise,
D.T.
Harris.
Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy.
2013;
15
:
330-343
.
-
A.
Eirin,
X.Y.
Zhu,
C.M.
Ferguson,
S.M.
Riester,
A.J.
van Wijnen,
A.
Lerman,
L.O.
Lerman.
Intra-renal delivery of mesenchymal stem cells attenuates myocardial injury after reversal of hypertension in porcine renovascular disease. Stem cell research & therapy.
2015;
6
:
7
.
-
S.
Francois,
M.
Mouiseddine,
B.
Allenet-Lepage,
J.
Voswinkel,
L.
Douay,
M.
Benderitter,
A.
Chapel.
Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects. BioMed research international.
2013;
2013
:
151679
.
-
H.J.
Harn,
S.Z.
Lin,
S.H.
Hung,
Y.M.
Subeq,
Y.S.
Li,
W.S.
Syu,
D.C.
Ding,
R.P.
Lee,
D.K.
Hsieh,
P.C.
Lin.
Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell transplantation.
2012;
21
:
2753-2764
.
-
N.J.
Kassebaum,
M.
Arora,
R.M.
Barber,
Z.A.
Bhutta,
J.
Brown,
A.
Carter,
D.C.
Casey,
F.J.
Charlson,
M.M.
Coates,
M.
Coggeshall.
Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet.
2016;
388
:
1603-1658
.
-
P.R.
Koppula,
L.K.
Chelluri,
N.
Polisetti,
G.K.
Vemuganti.
Histocompatibility testing of cultivated human bone marrow stromal cells - a promising step towards pre-clinical screening for allogeneic stem cell therapy. Cellular immunology.
2009;
259
:
61-65
.
-
Y.
Matsuda-Hashii,
K.
Takai,
H.
Ohta,
H.
Fujisaki,
S.
Tokimasa,
Y.
Osugi,
K.
Ozono,
K.
Matsumoto,
T.
Nakamura,
J.
Hara.
Hepatocyte growth factor plays roles in the induction and autocrine maintenance of bone marrow stromal cell IL-11, SDF-1 alpha, and stem cell factor. Experimental hematology.
2004;
32
:
955-961
.
-
I.
Mederacke.
Liver fibrosis - mouse models and relevance in human liver diseases. Zeitschrift fur Gastroenterologie.
2013;
51
:
55-62
.
-
G.B.D.
Mortality,
C.
Causes of Death.
Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet.
2015;
385
:
117-171
.
-
N.H.
Nicolay,
R.
Lopez Perez,
J.
Debus,
P.E.
Huber.
Mesenchymal stem cells - A new hope for radiotherapy-induced tissue damage?. Cancer letters.
2015;
366
:
133-140
.
-
B.
Parekkadan,
D.
van Poll,
Z.
Megeed,
N.
Kobayashi,
A.W.
Tilles,
F.
Berthiaume,
M.L.
Yarmush.
Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochemical and biophysical research communications.
2007;
363
:
247-252
.
-
A.
Reinisch,
N.
Etchart,
D.
Thomas,
N.A.
Hofmann,
M.
Fruehwirth,
S.
Sinha,
C.K.
Chan,
K.
Senarath-Yapa,
E.Y.
Seo,
T.
Wearda.
Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood.
2015;
125
:
249-260
.
-
D.
Schuppan,
N.H.
Afdhal.
Liver cirrhosis. Lancet.
2008;
371
:
838-851
.
-
A.
Seki,
Y.
Sakai,
T.
Komura,
A.
Nasti,
K.
Yoshida,
M.
Higashimoto,
M.
Honda,
S.
Usui,
M.
Takamura,
T.
Takamura.
Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology.
2013;
58
:
1133-1142
.
-
S.
Yuan,
T.
Jiang,
R.
Zheng,
L.
Sun,
G.
Cao,
Y.
Zhang.
Effect of bone marrow mesenchymal stem cell transplantation on acute hepatic failure in rats. Experimental and therapeutic medicine.
2014;
8
:
1150-1158
.
-
Y.
Zhang,
X.M.
Chen,
D.L.
Sun.
Effects of coencapsulation of hepatocytes with adipose-derived stem cells in the treatment of rats with acute-on-chronic liver failure. The International journal of artificial organs.
2014;
37
:
133-141
.
-
D.C.
Zhao,
J.X.
Lei,
R.
Chen,
W.H.
Yu,
X.M.
Zhang,
S.N.
Li,
P.
Xiang.
Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World journal of gastroenterology.
2005;
11
:
3431-3440
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 06 (2017)
Page No.: 1374-1387
Published on: 2017-06-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8478 times
- Download PDF downloaded - 2386 times
- View Article downloaded - 19 times
 Biomedpress
Biomedpress