Abstract
Introduction: Asphyxia is one of the important cause of infants' mortality. Accurate and early diagnosis of asphyxia has an important role in performing appropriate protective treatment protocole; therefore, we compared the diagnostic value of two methods of Prooxidant-Antioxidant Balance (PAB) and Heat shock proteins 70 ( HSP70) among healthy term infants and Neonates with asphyxia.
Methods: In this prospective case-control study, we compared the diagnostic value of two methods of PAB and HSP70 in healthy term infants (N=38) and Neonates with asphyxia (N=30) in Mashhad Ghaem hospital from 2011 to 2015. The diagnostic value of HSP70 and PAB was compared with statistical tests of Chi-square, T-Test, Man-Whitney, Roc curve and regression models.
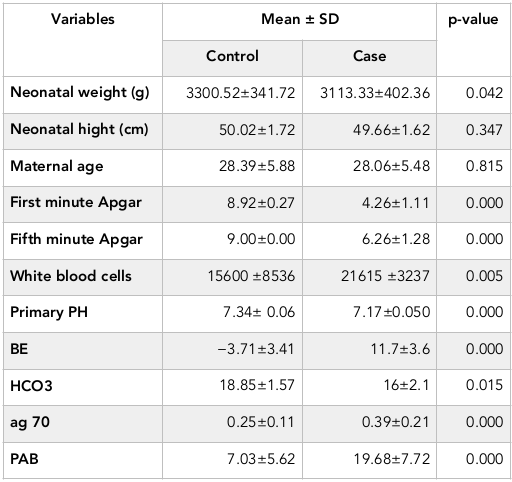
Results: The newborns in two groups were significantly different in terms of the first (P=0.000) and fifth minute Apgar score (P=0.000), HSP70 (P=0.000), PAB (P=0.000), PH (P=0.000), BE (P=0.000) and HCO3 (P=0.015). HSP>0.218 ng / dl has 60% sensitivity and 76% specificity for the diagnosis of asphyxia while PAB>11.3 HK has 84% sensitivity and 92% specificity for the diagnosis of asphyxia.
Conclusion: According to the results of this study, HSP70>0.22 ng/dl and PAB>11.3 HK Unite can be used as biochemical markers for the diagnosis of perinatal asphyxia (P=0.001). The sensitivity and specificity of PAB in the diagnosis of asphyxia is higher than HSP70 and Simultaneous measurement of these two markers can correctly diagnose 84% of asphyxia cases.
Introduction
The term asphyxia, is used to describe the interrupted supply of oxygen through umbilical cord to the fetus Boskabadi et al., 2015. It is estimated that 2 to 4 of every 1,000 term newborns suffer from birth asphyxia. Approximately, 15 to 33% of infants with asphyxia who show the symptoms of hypoxic ischemic encephalopathy (HIE) die during the neonatal period. 25% of survivors suffer from permanent neurological and psychological damages Ceccon, 2003Volpe, 1995. Asphyxia is defined as the signs of neonatal encephalopathy (hypotonia, reduced reflexes, the pupils' status, and seizure), first minute Apgar score of less than 4 and the fifth minute Apgar score of less than 7 and or umbilical pH of less than 7. Neonatal asphyxia is a serious and prevalent problem in prenatal cares Ceccon, 2003Kliegman, 2011Menkes H., 2006Volpe, 1995.
Asphyxia affects many major organs of the infant, but one of the irreversible and serious effects is its impact on the central nervous system. It leads to hypoxic ischemic encephalopathy, cerebral palsy, seizure and loss of learning AshrafganjueeWaldemar, 2011. In a study in Iran, asphyxia was reported the reason of 31% of infant mortality that means asphyxia is the second cause of neonatal death after severe prematurity Boskabadi H., 2010.
Although recognition of causing mechanisms and complications of asphyxia developed lately but the early diagnosis of brain damage following hypoxic-ischemic events is one of the most difficult problems in neonatal care yet Marlow, 2012Perlman, 1999Volpe, 1995. There is no specific reliable marker which is correlated with the extent of the damage of intrauterine hypoxia, hereupon diagnosis is usually based on non-specific clinical criteria. In the examination of neonates with asphyxia, usually no specific finding is obtained and it is suggested in differential diagnosis of other diseases such as sepsis, metabolic disorders, congenital metabolic defects, and so on Kliegman, 2011Low, 1997Siciarz et al., 2001. Parameters that are currently used for predicting or determining of perinatal asphyxia are as following: Apgar score, excessive umbilical arterial acidemia, and fetal electrical monitoring in the scalp with the presence of meconium. These findings are mostly non-specific and may have abnormal results when there is no major brain damage Aly et al., 2009Ashrafganjuee, 2004Boskabadi H., 2010Ghosh et al., 2003Kliegman, 2011Low, 1997Marlow, 2012Perlman, 1999Siciarz et al., 2001Waldemar, 2011.
Some researchers have applied biochemical (increased lactate, LDH: Lactate dehydrogenase, creatine kinase in the plasma, protein S100B, the retinol binding protein) and hematologic markers (NRBC count in umbilical vein blood) for the early detection of this damage Banupriya et al., 2008Basu et al., 2008Boskabadi et al., 2010Chen et al., 2000Ghosh et al., 2003.
Definite diagnosis, leading to differentiate asphyxia from more treatable problems and improve prognosis of clinical and neurological status of the newborn. In addition to the above, cerebral hypothermia and antioxidant treatments are suggesting to limit the nerve complications caused by ischemic-hypoxic damage Cheng et al., 1997Gunn et al., 2005Kliegman, 2011. These treatments have most effectiveness when used in early stages.
Heat shock proteins (HSP) are present in all organisms and cell types. In stable condition, HSP serum levels are very low. In response to stress such as high temperature, free radicals, fast tension and toxins, cells release HSP family (24). Some researches revealed that HSP70 is an important factor in the correct and timely diagnosis of ischemic-hypoxic events Child et al., 2006Fiedorowicz et al., 2008Jiang et al., 2004Ozer et al., 2002.
Lack of reliable and accurate method in determining the balance between oxidants and antioxidants of the patients is a major limitation. Recently, with simple and inexpensive method 3, 3’, 5, 5'-tetramethylbenzidine (TMB), this problem is partially solved Alamdari et al., 2007Boskabadi et al., 2014. Regarding to this fact that was not find any study which compared diagnostic value of the two methods of Prooxidant-Antioxidant Balance (PAB) and Heat shock proteins (HSPs) in term newborns with and without perinatal asphyxia and due to the complications caused by asphyxia and early diagnosis importance, the researchers decided to perform this study to achieve a reliable and accurate method in proper diagnosis of asphyxia, and evaluate the diagnostic value of two new biochemical criteria too.
Materials - Methods
This prospective study was performed from December 2010 to April 2015 in Ghaem hospital, Mashhad, Iran. Among 80 evaluated neonates, 68 eligible infants enrolled to the study, and HSP70 and PAB were measured for them. This study was approved by Mashhad University of Medical Sciences Ethics Committee (900514 and 900660).
In an analytical-observational study, the researchers evaluated HSP70 and PAB in term infants with perinatal asphyxia and compared it with healthy term infants. The infants in case group had at least two of the following symptoms:
1. Signs of fetal distress (FHR <100, no heart rate variability, Late deceleration).
2. Thick meconium in addition to hypotonia or bradycardia or respiratory depression.
3. Apgar score less than 4 in first minute and less than 7 in fifth minutes.
4. The need to CPR more than one minute with IPPV and oxygen.
5. PH <7.2 or BE <-12 during the first 6 hours after birth in newborn.
The control group included term newborns who had normal pregnancy and vaginal delivery and had stable clinical status during the first week of birth. Exclusion criteria in the case group included the infants with congenital abnormality and infection, sepsis, hypothermia, hypoglycemia, congenital heart disease and primary neurologic disorder. In control group neonate hospitalization during the first week and maternal problems during pregnancy or delivery were exclusion criteria.
The required data were collected through a researcher-made questionnaire covering information related to mothers (age, parity and method of delivery) and babies (gestational age, sex, birth weight, length, head circumference, length of stay, Apgar score).
The infants who were recruited to the study during the first day were completely examined by a neonatologist. In the third and seventh days, examination was repeated and the checklist was filled. The criteria to determine the severity of asphyxia was based on Sarnat clinical staging. In the examination, the infant's neurological function in the first, third and seventh days was evaluated by the examiner. This evaluation was as follows: Consciousness status, function of cranial nerve and movement and sensory systems. In the movement system examination, muscular tone and spontaneous movements of the infants were evaluated. Posture and muscles' strength vs. passive movements were examined to evaluate active tone.
In the infants of case group, the severity of HIE was determined based on Sarnat clinical staging. Mild HIE or HIE grade 1 defined as excessive vigilance, irritability and hyper reflex and no seizures for at least 24 hours after birth. The case of being lethargic, hypotonia and decreased reflexes, miotic pupils and seizures was considered as moderate HIE or HIE grade 2 and the case of apnea, stuporous, flaccid without primitive reflexes, severe convulsions or coma was considered as sever HIE or HIE grade 3.
Asphyxia complications such as breathing, cerebral, cardiac, gastrointestinal and kidney problems were monitored and recorded during the study. Evaluation of the patients was performed based on clinical examination, and laboratory required evaluation, and if there were medical indications, it was performed by imaging methods such as CX Ray, abdominal sonography and Brain CT Scan. In both of the control and experimental group after obtaining the written consent of the parents 2 ML of umbilical cord blood was collected and delivered in sterile tubes. After being allowed to clot, the tubes were centrifuged at 1000 rpm at room temperature to obtain serum. Hemolytic samples were excluded from analysis. Serum was stored at -70°C prior to analysis.Quantitative analysis (HSP70) and measurement of oxidant-antioxidant balance was performed in Department of Biochemistry of Bouali Research Institute. Serum Hsp 70 antigen concentrations were determined using a sandwich ELISA (The enzyme-linked immunosorbent assay) in-house. After overnight incubation 100 μL monoclonal Hsp70 antibody at 4°C, the plate was washed and non-specific binding sites blocked by incubation with 0.1% BSA. Plates were washed and 100 μL of standards, 5000 ng/mL of recombinant Hsp70, and undiluted serum were incubated for 2 hours at 37°C. After adding 100 μL of rabbit polyclonal anti-Hsp70, 100 μL of an anti-rabbit immunoglobulin peroxidase conjugate was added to the plate for 1 hour at 37°C. After adding 100 μl of TMB substrate, the reaction was stopped after 20 minutes with 2 M HCl and the absorbance read at 450 nm. The sensitivity of the assay was 39 ng/mL, and the inter- and intra-assay coefficient of variation was 9% and 6% respectively.
Finally, a suitable substrate such as tetramethyl benzidine creates blue color that changes to yellow color by the two normal chloridric acid which has absorption at wavelength of 450 nm. The different concentrations of the antigen is a result of the rate of absorption.
TMB determined Oxidant-antioxidant balance in two different reactions: in enzyme reaction chromogen is oxidized by peroxides (in this test, H2O2) to TMB containing cation. In latter reaction TMB containing cation is resuscitated by antioxidants (in this test, uric acid). During six stages with certain concentrations, a standard curve is obtained. This curve determines concentration of serum samples with detectable absorptions at 450 nm wavelength and calculate the oxidant-antioxidant balance. The standard solutions were prepared by mixing varying proportions (0–100 %) of 250 μM hydrogen peroxide with 3 mM uric acid (in 10 mM NaOH). For the preparation of the TMB cation, 60 mg TMB powder was dissolved in 10 mL DMSO; then 400 μL of TMB/DMSO was added in 20 mL of acetate buffer (0.05 M buffer, pH 4.5), and then 70 μL of fresh chloramine T (100 mM) solution in distilled water was added into this 20 mL, mixed well, incubated for 2 h at room temperature in a dark place; 25 units of peroxidase enzyme solution was added into 20 mL TMB cation, dispensed in 1 mL and stored at −20 °C. In order to prepare the TMB solution, 200 μL of TMB/DMSO was added into 10 mL of acetate buffer (0.05 M buffer, pH 5.8); the working solution was prepared by mixing 1 mL TMB cation with 10 mL of TMB solution, incubated for 2 min at room temperature in a dark place and immediately used.
Ten microliters of each sample, standard or blank (distilled water) were mixed with 200 μL of working solution, in each well of a 96 well plate, which was then incubated in a dark place at 37 °C for 12 min; at the end of the incubation time, 100 μL of 2 N HCl was added to each well; and measured in an ELISA reader at 450 nm with a reference wavelength of 620 nm. A standard curve was constructed from the values derived using standard samples. The values of the PAB were expressed in arbitrary HK units, which represent the percentage of hydrogen peroxide in the standard solution. The values of the unknown samples were then calculated based on the values obtained from the standard curve.
Data analysis and statistical analysis
All statistical analyses were performed with Statistical Package for the Social Sciences 15 (SPSS Science, Apache Software Foundation, and Chicago, IL, USA). Values were expressed as mean ± SD. Student t test, Kruskal-Wallis test and Mann–Whitney test were used as appropriate. Parametric and non-parametric correlations were assessed using Pearson correlation coefficients and Spearman correlation coefficients, respectively. P<0.05 was considered significant. Roc curve to compare the diagnostic value of HSP70 and PAB in the diagnosis of asphyxia it was plotted to calculate sensitivity and specificity of the test and comparing with conventional methods, after that regression methods to process the appropriate model of comparison of HSP70 and PAB in neonatal asphyxia.
Results
Thirty neonates in the case group and 38 neonates in the control group were studied. Forty percent of cases were female and 60 % were male. 42% of controls were female and 58% were male (P=0.861).
Sex, height, gestational age and maternal age in two groups are homogeneous (P>0.05, Table 1 ). First and fifth minute Apgar score, HSP70, PAB, PH, BE and HCO3 in two groups had significant statistical difference (P<0.05, Table 1 ).
In case group, 9 infants had seizures, 4 cases died and 26 have been survived. In terms of grading HIE in case group, 21 infants catch Ischemic-Hypoxic Encephalopathy grade 1, 8 cases of grade 2 and 1 case of grade 3 HIE.
Mean amount of PAB in normal infant, hypoxic-ischemic grade 1 and grade 2 include 7.03 HK, 19.4 HK and 21.6 HK, respectively (P=0.000). Mean of HSP70 in normal infant was 0.25 ng/dl, and in hypoxic-ischemic grade 1 was 0.38 ng/dl, and in grade 2 was 0.45 ng/dl (P = 0.005). Significant correlation was between HSP70 and PAB at the 0.01 level (Pearson correlation=0.392).
In case group, 90% of infants had PAB>11.01 that this rate was 13.2% in the control group, and two groups showed a significant difference in terms of PAB levels (P=0.000).
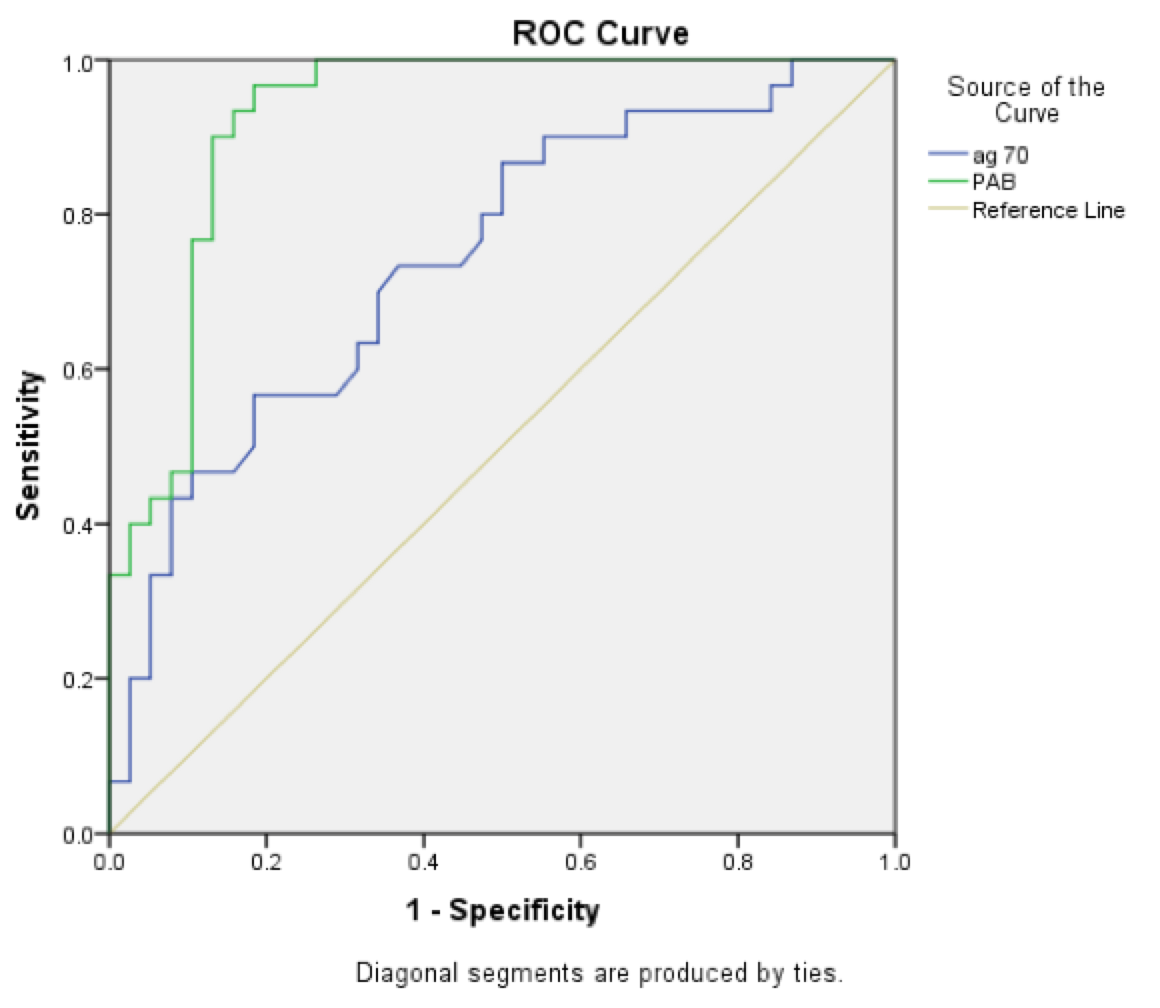
Fig. 1 illustrates the sensitivity and specificity of the two criteria of PAB and ag70 for the diagnosis of asphyxia. The amount of HSP>0.218 has 60% sensitivity and 76% specificity for the diagnosis of asphyxia, while PAB>11.3 has 84% sensitivity and 92% specificity for the diagnosis of asphyxia. The values of PAB and ag70 have significant statistical difference between case and control groups (P=0.001). It seems that specificity and sensitivity of PAB is more than ag70 for the asphyxia diagnosis ( Figure 1 ).
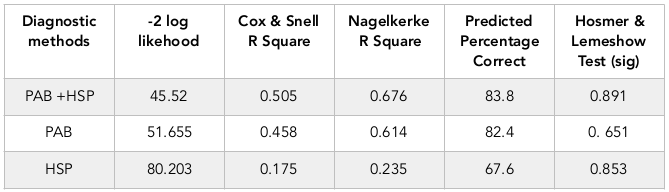
Table 2 exhibits predictive values of HSP70 and PAB alone or together. As seen in the table, considering of HSP70 and PAB can distinct 83.8% cases of asphyxia correctly.
Discussion
According to the results of this study, although measurements of both laboratory markers of ag70 and PAB is effective in the diagnosis of neonatal asphyxia, but the value of PAB has more specificity and sensitivity than the value of HSP70, and measurement of both of these markers in the same time can detect 84% cases of asphyxia correctly.
In the current study, the first and fifth minute Apgar had significant statistically difference in the two groups. In the Bijari study, the neonatal parameters such as fifth minute Apgar score, PH and bicarbonate were less in the infants with asphyxia than healthy infants Bahman Bijari et al., 2010. In the Hogan study , all formal criteria are fulfilled in 10% of asphyxia, and they have observed Apgar 4-6 in prediction of half of the asphyxia Hogan et al., 2007. Conventional Apgar score cannot predict the status of the infant in asphyxia. It neglects prematurity characteristics and the course of resuscitation measures. Indeed fifth Apgar scores and even 10 minute of undergoing resuscitation or ventilated neonates have no value for evaluating asphyxia and predicting other problems associated with it. Regarding above Rüdiger and his colleagues in one of their studies reported that the value of Apgar is limited for the infants who are premature and survived Rudiger, 2008. Low Apgar score alone is inappropriate to assess asphyxia" Lopriore et al”said Lopriore et al., 2004.
According to the results of this study control and patient groups have significant difference in terms of the amount of HSP70 antigen (P=0.001). The HSP70 antigen rate is much higher in the patient group. Against environmental stress, cells function such as the synthesis of DNA, RNA and protein is decreased or completely impaired. In such circumstances, certain proteins usually appear in the cells which are called protein stress. HSP70 is one of stress proteins that play an important role in protecting cells against stress and injuries. These proteins identify damaged molecules and divide them into two categories of repairable and unrepairable. After that it bind to damaged and repairable proteins hold them steady until the cell is repaired and obtain the energy to construction. HSP70 inhibits apoptosis and cell death in a complex mechanism. Asphyxia is a type of environmental stress that in different animal studies its relation with HSP70 has been examined. Dr. Ozer express after the hypoxic stress, HSP70 increases more in 12 days mice (equivalent to term infant) than 7 days mice (human fetus with 32 to 34 weeks gestational age) (P=0.003). Above results frame an important role for HSP70 in response to hypoxic stress in older newborns Ozer et al., 2002.
Chen study was about the regulation effects of memantine on the synthesis and expression of HSP70 gene following hypoxia and ischemia in rats' infants. He exposed to discussion that the synthesis and expression of HSP70 gene is increased in hypoxia and ischemia and can be a sensitive marker in this relation Chen et al., 2003. Also, levels of HSP70 and then HSP27 are increased following hypoxia and ischemia in the cortex and hippocampus of mice significantly (P<0.05) Jiang et al., 2004. In the Cheng study on 24 pigs at third day of birth, it was found that after hypoxic-ischemic encephalopathy, expression of HSP70 after the third hours began to increase and reaches its maximum at 6th hour (P<0.01) Cheng et al., 2005. Despite these animal studies, the researcher only found just a human study on the role of HSP 70 in the neonatal asphyxia. In a study in 2015 that has compared the amount of HSP70 in 51 neonates with asphyxia and 50 healthy infants showed that mean level of HSP 70 in neonates with asphyxia was 0.36 ng/mL and in healthy infants was 0.24 ng/mL (P=0.001) Boskabadi et al., 2015.
The results of current study show that the HSP> 0.218 ng/dl has 60% sensitivity and 76% specificity for the diagnosis of asphyxia. In another study, this antigen had 58% sensitivity and 73% specificity for the diagnosis of asphyxia, which means that if it was positive, it is highly suggestive of the diagnosis of asphyxia, but if negative, the diagnosis of asphyxia cannot be denied base on it Boskabadi et al., 2015. Therefore, it seems that despite helping to diagnose asphyxia, it cannot be used for definite diagnosis alone, and it is better to use it along with other criteria.
We have found that PAB values were significantly higher in neonates with perinatal asphyxia. According to the results of our study, the PAB in neonates with asphyxia was about three times more than normal infants (P=0.000). In a study conducted by Ashok Kumar and colleagues, the level of oxidative stress in perinatal asphyxia has been studied. Plasma malondialdehyde levels and malondialdehyde of cerebrospinal fluid and ratio of plasma / cerebrospinal fluid of malondialdehyde were significantly higher in the infants with asphyxia in comparison with the infants in control group (62.5 vs. 88.2 mmol/L in plasma). Excessive production of free radicals of oxygen and lipid peroxidation is actors who play important roles in perinatal asphyxia Kumar et al., 2008. In another study, the value of PAB was significantly higher in the infants with asphyxia than healthy infants that is consistent with the present study Boskabadi et al., 2014. There is a critical balance in cells between the formation of free radicals and antioxidant defense and restorative systems; it means that in physiological conditions, there is a balance between antioxidants and peroxidants. In normal condition free radicals are neutralized by antioxidant system. Hypoxic conditions like as birth asphyxia increase the production of free radicals in the blood and cells, thus balance of peroxide-antioxidant is impaired Boskabadi et al., 2014. Stressful conditions such as hypoxia stimulates the production of free radicals by reduction in oxidation and phosphorylation pairing in the mitochondria it leads to increased leakage of electrons and excessive production of superoxide radicals. When the production of free radicals was more than the capacity of antioxidant system for the neutralization, lipid peroxidation damage to the unsaturated lipids in the cell membrane, amino acids in proteins and nucleotides in the DNA.
As a result, the integrity of cell and membrane is impaired. This status will be more severe by reduced the efficiency of the immune system and unfavorable changes in the cardiovascular system, brain and nervous system and muscular system through increased lipid oxidation; therefore, many of the symptoms and complications of asphyxia may occur following the imbalances of the antioxidant-oxidant balance Boskabadi et al., 2014Surai, 2007.
According to the results of this study, PAB>11.3 HK Unite had sensitivity of 84% and specificity of 92% for the diagnosis of asphyxia that revealed it as a suitable factor for the diagnosis of asphyxia and has better sensitivity and a higher specificity compared to HSP70.
Finally, based on the results of current study, levels of HSP70>0.22 ng/dl and PAB >11.3 HK Unite can be suitable biochemical markers for the diagnosis of perinatal asphyxia (P=0.001). Although PAB has a greater value than HSP70 in the diagnosis of perinatal asphyxia and has higher sensitivity and specificity, but simultaneous use of these two factors at the same time can diagnose more than 84% of asphyxia cases properly.
Low cooperative parents and small sample subgroup of HIE was the major restriction in the current study. Further studies are needed to confirm the emerging data and value of PAB and HSP assay for identification of asphyxiated infants.
Conclusion
The PAB and HSP70 methods are rapid tests that may be useful for risk prediction in perinatal asphyxia when used with other forms of assessments e.g to consider first minute and fifth minute Apgar scores in judgment can distinguish among healthy and asphyxiated infants. The authors admit that in this study PAB shows high sensitivity and specificity in comparison HSP70.In addition above gasometrical parameters such as HCO3, BE and Primary PH were statistically significant so they can help in the diagnosis. However, further clinical research is required on larger populations, as well as on various physiological and pathological correlates of oxidative stress and parameters of asphyxia.
Abbreviations
APX: asphyxia
CPR: Cardiopulmonary resuscitation
CT: Computed tomography
ELISA: The enzyme-linked immunosorbent assay
FHR: fetal heart rate
HCl: hydrogen chloride
HIE: hypoxic ischemic encephalopathy HSPs: heat shock proteins
IPPV: intermittent positive pressure ventilation
LDH: Lactate dehydrogenase
mL, ml, or mℓ: Milliliter
PAB: prooxidant-antioxidant balance pH: potential of hydrogen
Author contribution
Hassan Boskabadi conducted the research, Gholamali Maamouri performed data analysis, Maryam Kalate Mollaey drafted the manuscript, Majid Ghayour-Mobarhan reviewed the manuscript, Maryam Zakerihamidi supervised the study, and Fatemeh Bagheri, Elahe abbasi, Afsaneh zareh, Akram Tamannanlo sampling and collecting and recording data in statistical software.
References
-
D.H.
Alamdari,
K.
Paletas,
T.
Pegiou,
M.
Sarigianni,
C.
Befani,
G.
Koliakos.
A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clinical biochemistry.
2007;
40
:
248-254
.
-
H.
Aly,
S.
Hassanein,
A.
Nada,
M.H.
Mohamed,
S.H.
Atef,
W.
Atiea.
Vascular endothelial growth factor in neonates with perinatal asphyxia. Brain and Development.
2009;
31
:
600-604
.
-
Ashrafganjuee.
Umbilical cord entanglement and intrapartum complications during childbirth [Article in Persian]. J Shahrekord Univ Med Sci.
2004;
2
.
-
B.
Bahman Bijari,
Z.
Farahmandinia,
A.
Hazeghi.
Predictive Value of Nucleated Red Blood Cell Counts in Cord and Peripheral Blood of Asphyxiated Term Neonates in the First Week of Life. Journal of Shahid Sadoughi University of Medical Sciences.
2010;
17
:
330-336
.
-
C.
Banupriya,
P.
Doureradjou,
N.
Mondal,
B.
Vishnu,
B.
Koner.
Can urinary excretion rate of malondialdehyde, uric acid and protein predict the severity and impending death in perinatal asphyxia?. Clinical biochemistry.
2008;
41
:
968-973
.
-
P.
Basu,
S.
Som,
N.
Choudhuri,
H.
Das.
Correlation between Apgar score and urinary uric acid to creatinine ratio in perinatal asphyxia. Indian journal of clinical biochemistry.
2008;
23
:
361-364
.
-
H.
Boskabadi,
A.N.
Boroujeni,
H.
Mostafavi-Toroghi,
G.
Hosseini,
M.
Ghayour-Mobarhan,
D.H.
Alamdari,
M.
Biranvandi,
H.
Saber,
G.A.
Ferns.
Prooxidant-antioxidant balance in perinatal asphyxia. The Indian Journal of Pediatrics.
2014;
81
:
248-253
.
-
H.
Boskabadi,
G.
Maamouri,
M.H.
Sadeghian,
M.
Ghayour- Mobarhan,
M.
Heidarzade.
Early diagnosis of perinatal asphyxia by nucleated red blood cell count: a case-control study. Archives of Iranian medicine.
2010;
13
:
275
.
-
H.
Boskabadi,
M.
Omidian,
S.
Tavallai,
S.
Mohammadi,
M.
Parizadeh,
M.G.
Mobarhan,
G.A.
Ferns.
Serum Hsp70 antigen: Early diagnosis marker in perinatal asphyxia. Iranian journal of pediatrics.
2015;
25
.
-
H. P.Z.
Boskabadi,
T.
Barati,
A.
Moedi.
Predisposing factors to investigate the causes of infant mortality in the city of Mashhad Ghaem hospital [Article in Persian]. Iran J Obstet Gynecol infertile.
2010;
14
:
6-14
.
-
M.E.J.R.
Ceccon.
Interleukins in hypoxic-ischemic encephalopathy. Jornal de pediatria.
2003;
79
:
280-281
.
-
H.
Chen,
Z.
Liu,
Z.
Zhou,
M.
Jiang,
L.
Qian,
S.
Wu.
The regulatory effect of memantine on expression and synthesis of heat shock protein 70 gene in neonatal rat models with cerebral hypoxic ischemia. Chinese medical journal-Beijing-English edition.
2003;
116
:
558-564
.
-
H.-J.
Chen,
K.-I.T.
Yau,
K.-S.
Tsai.
Urinary uric acid/creatinine ratio as an additional marker of perinatal asphyxia. Journal-formosan medical association.
2000;
99
:
771-774
.
-
Y.
Cheng,
J.M.
Gidday,
Q.
Yan,
A.R.
Shah,
D.M.
Holtzman.
Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic—ischemic brain injury. Annals of neurology.
1997;
41
:
521-529
.
-
Y.
Cheng,
G.
Liu,
J.
Guan,
Y.
Guo,
Y.
Li,
R.
Wu.
Early diffusion weighted imaging and expression of heat shock protein 70 in newborn pigs with hypoxic ischaemic encephalopathy. Postgraduate medical journal.
2005;
81
:
589-593
.
-
D.F.
Child,
P.R.
Hudson,
C.
Hunter-Lavin,
S.
Mukhergee,
S.
China,
C.P.
Williams,
J.H.
Williams.
Birth defects and anti-heat shock protein 70 antibodies in early pregnancy. Cell stress & chaperones.
2006;
11
:
101-105
.
-
M.
Fiedorowicz,
D.
Makarewicz,
K.I.
Stańczak-Mrozek,
P.
Grieb.
CDP-choline (citicoline) attenuates brain damage in a rat model of birth asphyxia. Acta Neurobiol Exp.
2008;
68
:
389-397
.
-
B.
Ghosh,
S.
Mittal,
S.
Kumar,
V.
Dadhwal.
Prediction of perinatal asphyxia with nucleated red blood cells in cord blood of newborns. International Journal of Gynecology & Obstetrics.
2003;
81
:
267-271
.
-
A.J.
Gunn,
P.D.
Gluckman,
J.S.
Wyatt,
M.
Thoresen,
A.D.
Edwards,
C.S.
Group.
Selective head cooling after neonatal encephalopathy. The Lancet.
2005;
365
:
1619-1620
.
-
L.
Hogan,
I.
Ingemarsson,
K.
Thorngren-Jerneck,
A.
Herbst.
How often is a low 5-min Apgar score in term newborns due to asphyxia?. European Journal of Obstetrics & Gynecology and Reproductive Biology.
2007;
130
:
169-175
.
-
K.
Jiang,
C.
Yang,
Q.
Shui,
Z.
Xia,
Y.
Zhang.
Time-course of mu-calpain activation, c-Fos, c-Jun, HSP70 and HSP27 expression in hypoxic-ischemic neonatal rat brain. Zhonghua er ke za zhi= Chinese journal of pediatrics.
2004;
42
:
441-445
.
-
Kliegman.
Hypoxia-ischemia. In Nelson textbook of Pediatrics 19th ed, K.M. Behrman ER, Arvin AM, eds., ed.. Philadelphia: WB Saundres Company.
2011;
:
493-496
.
-
A.
Kumar,
S.V.K.
Ramakrishna,
S.
Basu,
G.R.K.
Rao.
Oxidative stress in perinatal asphyxia. Pediatric neurology.
2008;
38
:
181-185
.
-
E.
Lopriore,
G.F.
van Burk,
F.J.
Walther,
A.J.
de Beaufort.
Correct use of the Apgar score for resuscitated and intubated newborn babies: questionnaire study. Bmj.
2004;
329
:
143-144
.
-
J.A.
Low.
Intrapartum fetal asphyxia: definition, diagnosis, and classification. American journal of obstetrics and gynecology.
1997;
176
:
957-959
.
-
Marlow.
Do we need an apgar score?. Arch Dis child.
2012;
67
:
765-767
.
-
H. S.H.
Menkes.
Perinatal asphyxia and trauma. In Child Neurology (Philadelphia: MD:Williams and Wilkins).
2006
.
-
E.
Ozer,
O.
Yilmaz,
M.
Akhisaroglu,
B.
Tuna,
A.
Bakiler,
E.
Ozer.
Heat shock protein 70 expression in neonatal rats after hypoxic stress. The Journal of Maternal-Fetal & Neonatal Medicine.
2002;
12
:
112-117
.
-
J.M.
Perlman.
Markers of asphyxia and neonatal brain injury. Mass Medical Soc.
1999
.
-
Rudiger.
Trial to Evaluate a Specified Type of APGAR. 2008
.
-
A.
Siciarz,
B.
Weinberger,
G.
Witz,
M.
Hiatt,
T.
Hegyi.
Urinary Thiobarbituric Acid-Reacting Substances as Potential Biomarkers of Intrauterine Hypoxia. Archives of pediatrics & adolescent medicine.
2001;
155
:
718-722
.
-
P.F.
Surai.
Natural antioxidants in poultry nutrition: new developments. Paper presented at: Proceedings of the 16th European symposium on poultry nutrition (World Poultry Science Association).
2007
.
-
J.
Volpe.
Neurology of the newborn 3rd ed. WB Saunders, Philadelphia.
1995
.
-
Waldemar.
Nelson textbook of pediatrics. Philadelphia: Saunders.
2011
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 05 (2017)
Page No.: 1327-1340
Published on: 2017-05-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 6574 times
- Download PDF downloaded - 2262 times
- View Article downloaded - 7 times
 Biomedpress
Biomedpress