Abstract
Introduction: Hemostasis is a process which preserves the stability of a closed and high-pressure circulatory system after any vascular injury. Circulating platelets are recruited to the site of injury, where they develop a major component of the developing thrombus, blood clotting, started by tissue factor, concludes in the generation of thrombin and fibrin. Thrombosis is a serious event in the arterial diseases and a major cause in the development of myocardial infarction, stroke and venous thrombo-embolism which justify prominent morbidity and mortality rate. The knowledge of molecular and cellular mechanism of the formation of thrombus has developed considerably in the recent studies by using different in-vitro and in-vivo models of diseases. P. gerardiana nut oil has been reported to possess anti-bacterial, anti-fungal, anti-viral, anti-septic, anti-neuralgic, diuretic, expectorant, hypertensive properties. However, hardly, any data is available regarding effects of nut oil on platelet function. In this study, fibrinolytic activity and effect on platelet aggregation were investigated. Method: P. gerardiana nut oil was extracted by using n-Hexane and then concentrated by rotary evaporator. Anti-thrombotic and fibrinolytic activities were evaluated on blood clot formation. Effects on platelet aggregation of the oil were determined based on collagen or epinephrine induced platelet aggregation. Results: P. gerardiana caused blood clot lysis in-vitro. P. gerardiana nut oil inhibited collagen dependent platelet aggregation while accelerated the epinephrine dependent platelet aggregation. In vitro whole blood coagulation was also reduced. In vivo P. gerardiana nut oil has no significant effect on blood cell indices. Conclusion: P. gerardiana nuts oil can be an effective therapy for the treatment of cardiovascular disorders and thromboembolism.
Introduction
Thrombosis is local coagulation or clotting of the blood in a part of the circulatory system. A more balanced clot is called a thrombus, must develop a seal which is long lasting on large damaged blood vessels. The clotting procedure is self-restricting and anti-thrombin prevent the clot from continuous enlargement, otherwise the developed clot block the blood flow in the vessel. Thrombosis (excessive blood clot formation) within healthy blood vessels can be harmful if not treated within time. Cardiovascular diseases like heart attack, stroke and pulmonary embolism and several others have been related to inappropriate blood clot formation Furie and Furie, 2008. Synthetic drugs show many side effects and require closed monitoring e.g., tranexamic acid (anti-fibrinolytic drugs) and vitamin K has been used in the treatment of bleeding disorder Sànchez-Lamar et al., 1999.
Platelets have important role in blood haemostasis as well as in pathogenesis of cardiovascular diseases Campbell et al., 2008Elliott et al., 1997. Platelet aggregation blood test (PABT) checks how well platelets, clump collectively and cause blood to become a clot Elliott et al., 1997. Blood clot is the aggregation of platelets, red blood cells and fibrin formation through a specific pathway that helps maintain hemostasis. When injury of blood vessel wall occurs, clot formation is completed within 5 minutes in a healthy person Sànchez-Lamar et al., 1999.
Chilgoza pine nuts (Pinus gerardiana) are the edible seed of the Chilgoza pine plant which belong to pinaceae family commonly found in Pakistan, India, China and Afghanistan Destaillats et al., 2010. P. gerardiana nuts contain un-saturated fatty acids which are very beneficial for lowering high cholesterol level. P. gerardiana nuts are good source of energy (628 kcal) and nutrition consisting of protein (11.6g/100g), carbohydrates (19.3g/100g) and fatty acids (61g/100g) Brufau et al., 2006. Regarding nutrition, P. gerardiana nuts contain vitamins, beta-carotene, thiamin (B1), riboflavon (B2), niacin (B3), pantothenic acid (B5), vitamin B6, folate (B9), vitamin C, vitamin E, vitamin k and minerals including calcium, iron, magnesium, manganese, phosphorus, zinc, potassium and phosphorus other constituents including water, so it can be used for both curative & nutritional purposes Sagrero-Nieves, 1992Savage, 2001.
P. gerardiana nut oil has anti-bacterial, anti-fungal, anti-viral, anti-septic, anti-neuralgic, choleretic, diuretic, expectorant, hypertensive properties Amr and Abeer, 2011Lawlees, 1992. Simillarly, hydro-alcoholic extract showed anti-inflammatory anti-bacterial and antioxidant properties Sharma et al., 2016.
Since oxidative stress contributes significantly in platelet activation Bartimoccia et al., 2015 and also synthetic anti-inflammatory drugs like aspirin has antiplatelet activity Roth and Majerus, 1975, the objectives of this study was to investigate the fibrinolytic activity and effect on platelet aggregation.
Materials-Methods
Preparation of Pinus gerardiana nuts oil concentrates (PGNC) Pine nuts were purchased in March 2015 from a local market in Lahore-Pakistan. After removing impurities and broken kernels, pine nuts were crushed into powder. P. gerardiana nuts oil was extracted using soxholet apparatus (jisico Model No:GLHMP-F100) and N-hexane was used as extractor solvent. The extract was concentrated to a final volume of 350 ml using rotary evaporator at 50ºC at reduced pressure. Oil concentrate was stored in amber green air-tight container and placed in dark at 20ºC. The concentrate was used in all experiments without further dilution.
Preparation of platelet rich plasma and platelet aggregation
The research protocol was approved by the research ethics committee of Faculty of Pharmacy the University of Lahore. Blood was drawn into 15 ml falcon tube containing 1ml tri-sodium citrate (3.2%) solution from healthy volunteers after informed consents. Platelet rich plasma (PRP) was prepared as described in Yavasoglu et al., 2010 with minor modification. Briefly, citratated supplemented blood was centrifuged for 15 minutes at 1200 rpm in centrifuge machine (Neuation Model no: I fuge D06) and stored at 37ºC in water bath (jisico Model No: J-IWB-KOREA). Presence of platelets was confirmed by automated cell analyzer (Roche) according to standard laboratory procedure. 500µl PRP was taken in cylindrical cuvette provided with a magnetic stirrer at 700rpm. PRP was equilibrated for three min. 10 µl epinephrine (1mM) or 10 µl rat tail collagen (3mg/ml) were used as agonists to activate platelet aggregation. To evaluate the effect of extract PRP was pretreated with 2µl, 5µl or 10µl of PG nuts oil concentrate (PGNC). Aggregation time was compared with that of agonist epinephrine (adrenaline, Ameer Pharma Pakistan) or collagen (3mg/ml) alone. The process was performed in triplicate for at least three different blood samples.
Fibrinolytic activity
6ml blood was drawn from median cubital vein of healthy volunteers. 400μl blood was immediately taken in glass bottle (diameter 2 cm) with flat bottom. Blood was allowed to clot in the form of thin film at 37ºC for half an hour. Blood clot film was washed twice with 1ml normal saline. The blood clot were treated with PGNC (30ul or 60ul) diluted in 1ml normal saline to observe the fibrinolytic effect. Control samples contained normal saline only. Samples were incubated at for 1 hour at 37ºC. Glass bottles were removed and shake gently without disturbing the intact clot. 200µl blood suspension dilute with 1ml normal saline was transferred to 1 cm spectrophotometer cuvette and check the absorption (λ=550nm) by UV/Vis spectrophotometer (Model No: T 80) Umesh et al., 2014. 100μl of blood suspension was again taken into eppendorf tubes that contained 1ml hydrochloric acid (0.1N). Acid hematin was quantified at λ=550nm spectrophotometrically.
Formation of clot
This test was carried out to conclude whole blood clotting time and the effect on the fibrin clot formation. PGNC were tested at different concentration 20 µl, 30 µl and 40 µl. 9ml blood was taken from healthy volunteers. Blood was prevented from clotting by adding 1ml tri-sodium citrate (3.2%) solution. 200µl blood was incubated for 1 hour at 37ºC with 20 µl, 30 µl and 40 µl of PGNC. Normal saline used as control. Afterwards complete blood coagulation was observed by adding 50 µl of calcium chloride (100mM) solution. The time for the formation of clot was noted at 37ºC in water bath (jisico Model No: J-IWB-Korea) Al-Mamun et al., 2012.
In vivo experiment
Wistar rats (10 rats) with weight of 200-270g were purchased from the animal house of University of Lahore. All animal experiments were approved by animal research ethic committee of University of Lahore (fop-uol-2015-005). Animals were maintained in a controlled environment at a temperature of 22ºC, humidity 40-60%.
Animals were grouped as follows: Group A: (Control Group): rats were provided with normal Saline. Group B: Pinus gerardiana nuts oil concentrate by using oral gavage at doses of 200 µl/kg/ per day. Animals were dosed daily for 15 days. At the 16th day animals were euthanized underanesthesis and blood was withdrawn by cardiac puncture and stored in EDTA tubes (ethylene-diamine-tetra-acetic acid) for the complete blood count (CBC) quantification on automated cell analyzer (Roche) according to standard laboratory procedure.
Statistical analysis
Data is presented as mean±SD of at least three independent experiments. Paired t-test or one-way ANOVA with Bonferoni post hoc test was applied to evaluate and compare the significant difference among means. A p<0.05 was considered significant.
Results
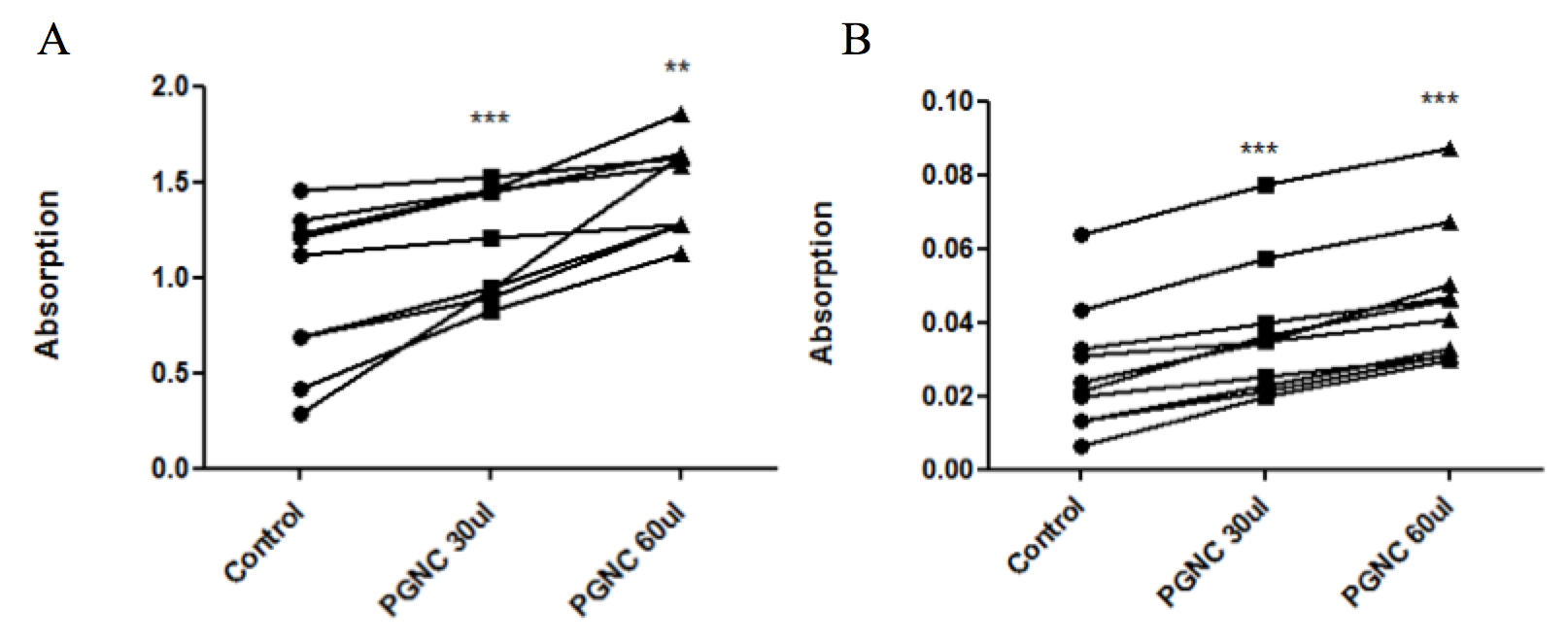
P. gerardiana induces Fibrinolysis (release of RBC) from a blood clot
The release of RBCs from the blood clot (as a measure of fibrinolytic activity) was measured. Absorption was directly proportional to the number of cells in the suspension. Figure 1 showed that P. gerardiana nuts oil increased clot lysis significantly in a dose dependent manner as compared with control (normal saline treated blood clots). The results were reassured by quantifying the haemoglobin (acid hematin) concentration of the suspension. The results showed that the acid haematin content also increased in the PGNC in a dose dependent way.
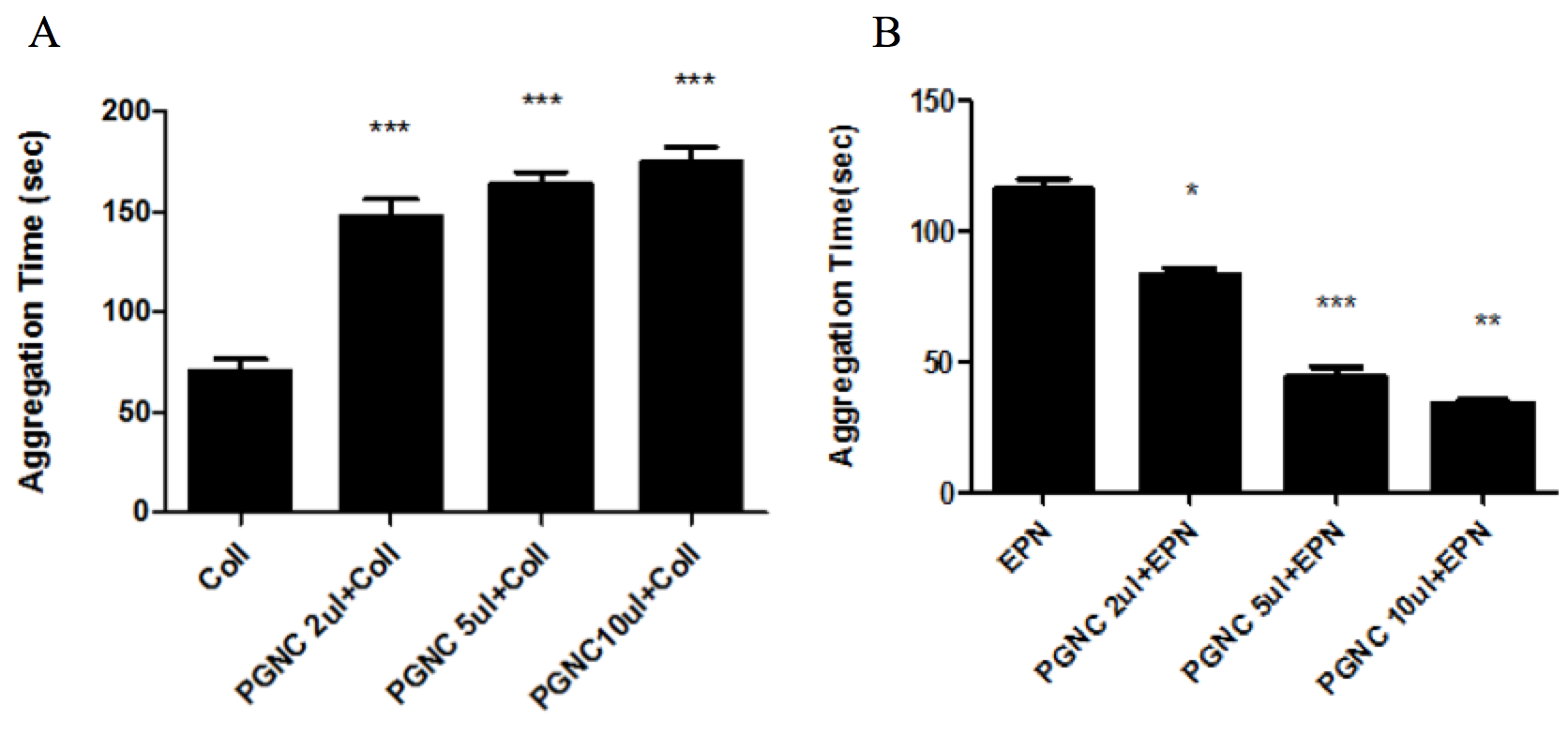
P. gerardiana has opposite effect on epinephrine and ollagen activated platelet aggregation
The effect of P. gerardiana nut oil (PGNC) on aggregation of platelet activated by collagen and epinephrine was tested. Platelets were incubated with different amounts of P. gerardiana and then activated by collagen or epinephrine. The control platelets were only treated with epinephrine (5µM) or 10µl rat tail collagen (3mg/ml). Treatment with PGNC showed opposite effects on the platelet aggregation activated by collagen or epinephrine ( Figure 2 ). PGNC significantly (p<0.001) and dose dependently increased the time for platelet aggregation when activated by collagen. This showed that the chemical constituents of PGNC interfere with the molecular mechanism of platelet aggregation induced or activated by collagen. The highest effect was observed when PRP was treated with 10µl PGNC. On the contrary, PGNC significantly (p<0.001) and dose dependently decreased the time for platelet aggregation when activated by epinephrine. These suggested that the chemical constituents mimic and facilitate platelet aggregation induction caused by epinephrine.
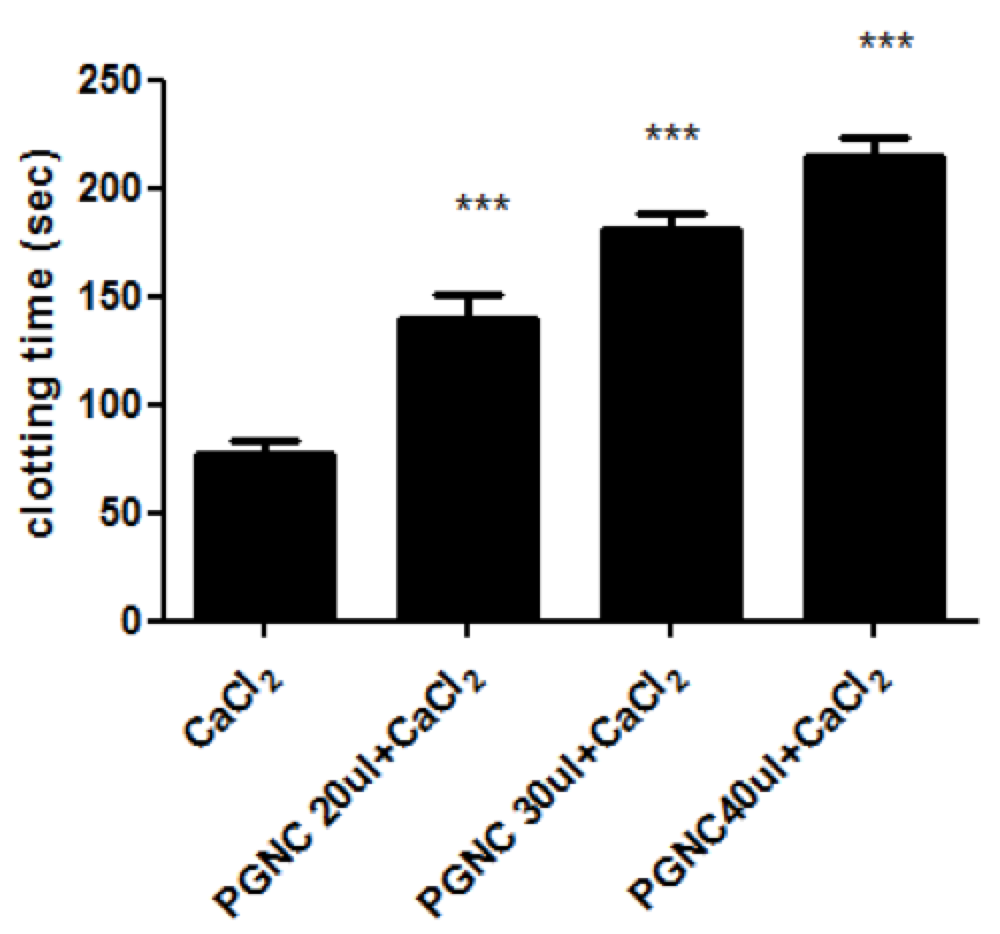
PGNC delays in-vitro Ca+2 stimulated blood clot formation
Blood clotting is spontaneous phenomenon. Many medications like aspirin can delay blood clot formation. The effect of PGNC was evaluated on in-vitro blood clot formation. The samples of blood were collected and clotting time was measured as shown in Figure 3 . The data showed that P. gerardiana nut oil concentrate (PGNC) significantly (p<0.01) and dose dependently (20µl, 30µl and 40µl) delayed the blood clotting time versus control. Normally, blood clotting was observed within 75 seconds after the addition of 50µl of CaCl2 (100 mM) solution that acted as control. Pre-treatment with 20µl PGNC showed around 90% increase in blood clotting time and the effect was even stronger at higher amounts of PGNC. The maximum effect was observed at 40µl PGNC that was about 3 times more than the control group.
PGNC does not reduce the platelet count
In order to check the effects of the PGNC on the blood cell indices, Wistar rats were treated with the concentrate 200mg/kg/day for 2 week. The blood was collected and analyzed for the complete blood count. No significant effect was observed on any blood indices parameter. Platelet, RBC, WBC counts were comparable to the control ( Figure 4 ).
Discussion
Platelets are blood cells that contribute in primary hemostasis. Primary hemostatic plug is formed by platelet-platelet interaction. Many studies have been carried out to develop anti-thrombotic agents with improved efficacy for preventing or treating arterial and venous thrombosis. The fibrinolytic enzyme prevents formation of fibrin clots in circulatory system. Some medicines like urokinase and streptokinase are widely used to inhibit homeostatic disorders, particularly thrombo emboli. The primary function of fibrinolytic activity is to disperse fibrin clot in a circulation. Alpha-2-anti-plasmin, plasminogen and tissue plasminogen activators all of which also play a vital role in clot lysis Biggs, 1972Li et al., 2010.
Current study describes the effect of pinus gerardiana nut concentrate (PGCN) on fibrinolytic activity and platelet aggregation in-vitro and in-vivo. P.gerardiana nut oil has increased the lysis of clot in comparison to control. Blood cells from fibrinolytic activity was observed by using a spectrophotometer ( Figure 1A ) The heme released from haemoglobin of red blood cells when 0.1N hydrochloric acid (HCl) was added to the solution of the red blood cells and then acid haematin was formed ( Figure 1B ). The release of RBCs and acid haematin were observed by spectrophotometry. In one of the study aqueous Rue extract was found effective as fibrinolytic effect by 12.53±5.67%. In our study, the fibrinolytic effect of P. gerardiana was found to be increased by 47% and 72%. A number of plant extracts and their products having fibrinolytic activity are recognized included Lumbricus rubellus, Pleurotus ostreatus, Spirodela polyrhiza, Flammulina velutipes, and Ganoderma lucidum, Ginger (Zingiber officinale), Garlic (Allium sativum) as well as from Bacillus sp. in Korean and Japanese fermented foods, chung kookjang, respectively Michelson et al., 2006.
The interactions between platelets and a variety of adhesive proteins, such as collagen, and soluble agonists, such as ADP supply potential targets for developing anti-platelet agents. Different therapies are available to prevent irregular activation and aggregation of platelets. Epinephrine is an adrenergic agonist, bind with alpha 2-adrenergic receptors on platelets and also increases the effect of aggregation caused by other platelet agonists Porth, 2005Strukova, 2001. The capability of epinephrine induces the effect of aggregating agents on accumulation like fibrinogen binding, intracellular Ca2+ recruitment, granular relief and protein phosphorylation, and there are some factors correlate with platelet hyper-activity and resulting untimely coagulation of blood Choi, 2002Lanza et al., 1988. Similarly, collagen is the most thrombogenic component of the sub-endothelium Baumgartner and Haudenschild, 1972.
Following vascular damage, collagen is exposed to circulating platelets and both acts as a substrate for the adhesion of platelets Cowan et al., 1981Poole and Watson, 1995 and induces platelet activation Poole and Watson, 1995.
The main evidence suggests that two receptors are involved in the platelet response to collagen, integrin α2β1 acts to adhere platelets to collagen, allowing platelets to interact with the lower affinity receptor glycoprotein VI, which is mainly responsible for platelet activation Morton et al., 1989Santoro et al., 1991.
At lower concentrations, many of the effects of collagen are enhanced by its production of thromboxane A2 (TXA) Nakano et al., 1986Pollock et al., 1986Rittenhouse and Allen, 1982. The collagen-induced increase in [Ca2+] can be decreased by inhibiting the production of TXA via the pretreatment of platelets with cyclooxygenase inhibitors such as aspirin Nakano et al., 1986.
P. gerardiana nuts oil concentrate by using its different concentrations was tested for platelet aggregation in the presence of both epinephrine and collagen showed opposite effect on platelet aggregation time ( Figure 2A,B ). Previously unrefined methanolic extract of Nepeta juncea were examined for action against human platelet and have shown significant inhibitory effects on platelet aggregation Hussain et al., 2009. Also it is investigated that extracts of garlic, onions, nettle and alfalfa are proved to be the most potent inhibitors of platelet aggregation in vitro Pierre et al., 2005. Camomile, bramble are similarly powerful at repressing platelet accumulation in-vitro Pierre et al., 2005. Toona microcarpa Harms leaf (TMHE) extract in-vitro significantly inhibited platelet aggregation induced by thrombin, but not by ADP or collagen Choi, 2002. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats Mekhfi et al., 2006. In this study, opposite effects of PGNC could be due to specific interference of phytochemicals with collagen or epinephrine dependent cell signaling in platelets.
Hemostasis is a process that is divided into two stages: platelet aggregation and coagulation. P. gerardiana nuts oil increased clotting time in comparison to control which was induced by calcium chloride ( Figure 3 ). This suggests that PGNC can be used cardiovascular diseases for anticoagulation. In-vivo effects of PGNC on blood indices were analysised. The result showed that P. gerardiana had no effect on CBC parameters. Result interpreted that there was no significant effect on RBCS, WBC, HGB, HCT and other parameter. P. gerardiana nuts oil has slightly increased the effect of MCHC but other parameters did not show any significant effect.
Conclusion
In summary, our data strongly suggest that P. gerardiana nut oil can be used in the treatment of thromboembolic disorders and this protective mechanism is associated with increased fibrinolysis and inhibition of collagen-stimulated platelet aggregation.
Abbreviations
CBC: complete blood count; Coll: collagen; EDTA: ethylene-diamine-tetra-acetic acid; EPN: epinephrine; HCT: hematocrit; HGB: hemoglobin; MCH: mean corpuscular hemoglobin; MCV: mean corpuscular volume; PGNC: Pinus gerardiana nuts oil concentrates; PLT: Platelets; PRP: platelet rich plasma; RBC: red blood cells; WBC: white blood cells.
Author contribution
AUR give the concept, designed experiment, analyzed data and gave final approval of the manuscript and was the supervisor of the project. SN performed experiment, analyzed data and wrote draft of manuscript. MZ helped in data acquisition and experiment conditions optimization SSH and JA helped in editing and literature review for the paper AAZ did data acquisition, organization and data analysis and helped in write up of final draft.
References
-
M.R.
Al-Mamun,
N.
Amrin,
J.
Begum,
M.A.
Mazid.
Thrombolytic activity of some spices and plants available in Bangladesh. The Thai Journal of Pharmaceutical Sciences.
2012;
36
:
72-77
.
-
A.R.
Amr,
E.
Abeer.
Hypolipideimic and hypocholestermic effect of pine nuts in rats fed high fat, cholesterol-diet. World Applied Sciences Journal.
2011;
15
:
1667-1677
.
-
S.
Bartimoccia,
C.
Nocella,
D.
Pastori,
P.
Pignatelli,
R.
Carnevale.
Platelet Oxidative Stress and Antioxidant Nutrients. Journal of Vascular Medicine & Surgery 2014.
2015
.
-
H.R.
Baumgartner,
C.
Haudenschild.
Adhesion of platelets to subendothelium. Annals of the New York Academy of Sciences.
1972;
201
:
22-36
.
-
R.
Biggs.
Human blood coagulation, haemostasis and thrombosis. 1972
.
-
G.
Brufau,
J.
Boatella,
M.
Rafecas.
Nuts: source of energy and macronutrients. British Journal of Nutrition.
2006;
96
:
S24-S28
.
-
N.A.
Campbell,
J.B.
Reece,
L.A.
Urry,
M.L.
Cain,
S.A.
Wasserman,
P.V.
Minorsky,
R.B.
Jackson.
AP Edition Biology. Benjamin/Cummings.
2008
.
-
J.W.
Choi.
Incidence of nonresponsiveness to epinephrine in platelets from healthy humans. Acta haematologica.
2002;
108
:
106-108
.
-
D.H.
Cowan,
A.L.
Robertson,
P.
Shook,
P.
Giroski.
Platelet adherence to collagen: role of plasma, ADP, and divalent cations. British journal of haematology.
1981;
47
:
257-267
.
-
F.d.r.
Destaillats,
C.
Cruz-Hernandez,
F.
Giuffrida,
F.
Dionisi.
Identification of the botanical origin of pine nuts found in food products by gas− liquid chromatography analysis of fatty acid profile. Journal of agricultural and food chemistry.
2010;
58
:
2082-2087
.
-
W.H.
Elliott,
D.C.
Elliott,
J.R.
Jefferson,
J.
Wheldrake.
Biochemistry and molecular biology. Oxford University Press Oxford.
1997
.
-
B.
Furie,
B.C.
Furie.
Mechanisms of thrombus formation. New England Journal of Medicine.
2008;
359
:
938-949
.
-
J.
Hussain,
N.
Jamila,
S.A.
Gilani,
G.
Abbas,
S.
Ahmed.
Platelet aggregation, antiglycation, cytotoxic, phytotoxic and antimicrobial activities of extracts of Nepeta juncea. African Journal of Biotechnology.
2009;
8
.
-
F.
Lanza,
A.
Beretz,
A.
Stierle,
D.
Hanau,
M.
Kubina,
J.-P.
Cazenave.
Epinephrine potentiates human platelet activation but is not an aggregating agent. American Journal of Physiology-Heart and Circulatory Physiology.
1988;
255
:
H1276-H1288
.
-
J.
Lawlees.
The encyclopaedia of essential oils. Element Books Limited: Boston.
1992
.
-
H.
Li,
W.
Huang,
Y.
Wen,
G.
Gong,
Q.
Zhao,
G.
Yu.
Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis CH Wright. Fitoterapia.
2010;
81
:
1147-1156
.
-
H.
Mekhfi,
M.
ElHaouari,
M.
Bnouham,
M.
Aziz,
A.
Ziyyat,
A.
Legssyer.
Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation. Phytotherapy research.
2006;
20
:
135-139
.
-
A.D.
Michelson,
A.L.
Frelinger,
M.I.
Furman.
Current options in platelet function testing. The American journal of cardiology.
2006;
98
:
S4-S10
.
-
L.F.
Morton,
A.R.
Peachey,
M.J.
Barnes.
Platelet-reactive sites in collagens type I and type III. Evidence for separate adhesion and aggregatory sites. Biochemical Journal.
1989;
258
:
157-163
.
-
T.
Nakano,
A.
Terawaki,
H.
Arita.
Measurement of Thromboxane A2 Elevation of Ionized Calcium in Collagen-Stimulated Platelets with the Photoprotein, Aequorin. Journal of biochemistry.
1986;
99
:
1285-1288
.
-
S.
Pierre,
L.
Crosbie,
A.K.
Duttaroy.
Inhibitory effect of aqueous extracts of some herbs on human platelet aggregation in vitro. Platelets.
2005;
16
:
469-473
.
-
W.K.
Pollock,
T.J.
Rink,
R.F.
Irvine.
Liberation of [3H] arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochemical Journal.
1986;
235
:
869-877
.
-
A.W.
Poole,
S.P.
Watson.
Regulation of cytosolic calcium by collagen in single human platelets. British journal of pharmacology.
1995;
115
:
101-106
.
-
C.
Porth.
Pathophysiology: Concepts of altered health states, Vol 1. Lippincott Williams & Wilkins.
2005
.
-
S.
Rittenhouse,
C.
Allen.
Synergistic activation by collagen and 15-hydroxy-9 alpha, 11 alpha-peroxidoprosta-5, 13-dienoic acid (PGH2) of phosphatidylinositol metabolism and arachidonic acid release in human platelets. Journal of Clinical Investigation.
1982;
70
:
1216
.
-
G.J.
Roth,
P.W.
Majerus.
The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. Journal of Clinical Investigation.
1975;
56
:
624-632
.
-
L.
Sagrero-Nieves.
Fatty acid composition of Mexican pine nut (Pinus cembroides) oil from three seed coat phenotypes. Journal of the Science of Food and Agriculture.
1992;
59
:
413-414
.
-
A.
Sànchez-Lamar,
M.
Fiore,
E.
Cundari,
R.
Ricordy,
R.
Cozzi,
R.
De Salvia.
Phyllanthus orbicularis aqueous extract: cytotoxic, genotoxic, and antimutagenic effects in the CHO cell line. Toxicology and applied pharmacology.
1999;
161
:
231-239
.
-
S.A.
Santoro,
J.
Walsh,
W.
Staatz,
K.
Baranski.
Distinct determinants on collagen support alpha 2 beta 1 integrin-mediated platelet adhesion and platelet activation. Cell regulation.
1991;
2
:
905-913
.
-
G.P.
Savage.
Chemical composition of walnuts (Juglans regia L.) grown in New Zealand. Plant Foods for Human Nutrition.
2001;
56
:
75-82
.
-
A.
Sharma,
R.
Goyal,
L.
Sharma.
Potential biological efficacy of Pinus plant species against oxidative, inflammatory and microbial disorders. BMC Complementary and Alternative Medicine.
2016;
16
:
35
.
-
S.
Strukova.
Thrombin as a regulator of inflammation and reparative processes in tissues. Biochemistry (Moscow).
2001;
66
:
8-18
.
-
M.
Umesh,
C.
Sanjeevkumar,
B.
Hanumantappa,
L.
Ramesh.
Evaluation of in vitro anti-thrombolytic activity and cytotoxicity potential of Typha angustifolia L leaves extracts. Int J Pharm Pharm Sci.
2014;
6
:
81-85
.
-
I.
Yavasoglu,
B.
Acar,
G.
Kadikoylu,
Z.
Bolaman.
Platelet aggregation tests are affected in pseudothrombocytopenia. Laboratory Medicine.
2010;
41
:
483-485
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 1 (2017)
Page No.: 1098-1109
Published on: 2017-01-16
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7580 times
- Download PDF downloaded - 2578 times
- View Article downloaded - 26 times
 Biomedpress
Biomedpress