Abstract
Introduction: Hyperhomocysteinaemia (HHcy) is an established risk factor for cardiovascular, cerebrovascular, peripheral vascular diseases and neurodegenerative disease. The effect of this HHcy on vascular diseases could potentially cause vascular pathology features. Experimental studies have demonstrated that Hcy can be neurotoxic to brain, hippocampus area.
Methods: The present study was conducted to compare the possible neuroprotective effects of different herbal cocktail in HHcy-induced rats’ brain cerebrovascular dysfunction model. Rats were divided into nine groups: Group I - Controls received the same volume of saline solution (0.5 mL/100 g of body weight). Group II served as HHcy and received homocysteine 0.03 μmol/g of b.w. daily for 30 days. Group III served as HHcy and received homocysteine 0.03 μmol/g of b.w. + Artemisia Judaica extract (AJ) (50 mg/kg per oral by oral feeding needle with tuberculin syringe) daily for 30 days. Group IV served as HHcy and received homocysteine 0.03 μmol/g of b.w.+ Panax ginseng extract (PG) (50 mg/kg per oral by oral feeding needle with tuberculin syringe) daily for 30 days. Group V served as HHcy and received homocysteine 0.03 μmol/g of b.w. + Polygonum multiflorum extract (PM) (400 mg/kg per oral by oral feeding needle with tuberculin syringe) daily for 30 days. Group VI served as HHcy and received homocysteine 0.03 μmol/g of b.w. + AJ + PG with the same dose of previous group daily for 30 days. Group VII served as HHcy and received homocysteine 0.03 μmol/g of b.w. + AJ + PM with the same dose of the previous group daily for 30 days. Group VIII served as HHcy and received homocysteine 0.03 μmol/g of b.w. + PG + PM with the same dose of the previous group daily for 30 days. Group IX served as HHcy and received homocysteine 0.03 μmol/g of b.w. + AJ + PG + PM with the same dose of previous group daily for 30 days. The hippocampus of brain samples was collected at the end of the experiment and measuring oxidative stress markers (CAT, SOD, MDA and NO), inflammatory mediators (IL-6 and BDNF), histopathological examination and comet assay.
Results: Revealed data showed that the homocysteine induces SOD, CAT depletion, and an increase in AChE, MDA, NO, IL-6, and BDNF. A mixture of PG and PM or the individual treatments showed an ameliorative response for all parameters. In general, oxidative stress parameters, inflammatory mediator, neurotrophic factor, pathological examination and comet were degenerate against HHcy but did not differ significantly compared to AJ group.
Conclusion: Better physiological and histological characteristics were in PG and PM and their combination groups compared with HHcy and ameliorated nearly the control group.
Introduction
Homocysteine (Hcy) is sulphur-containing amino acid derivative which causes damage to the endothelial cells of blood vessels and leads to atherosclerosis and vascular disorder Kumar et al., 2008. Hyperhomocysteinaemia (HHcy) is recognised as one of the major risk factors for stroke and cerebrovascular disease Boysen et al., 2003. This is supposed by the observation of McCully (1969) on autopsy of two infants born with homocysteinuria. They had died due to atherosclerosis of most of their blood vessels and myocardial disorder. These two infants had elevated levels of homocysteine, suggested cause of their death Sainani et al., 2007. HHcy also acts as a cofactor in many cardiovascular, neurovascular and renal diseases; it causes remodeling of blood vessels and affects blood-brain barrier (BBB) permeability Kumar et al., 2008.
Panax ginseng (pg) is widely used as a traditional herbal medicine. As major class of active ingredient that is responsible for the physiological activity of ginseng is the ginsenosides. It is not a single compound but a collection of different components Algohary et al., 2016. Various studies recorded the beneficial effects of ginseng; constriction of toxins uptake, inhibition of excitotoxicity, reduction of oxidative stress, by controlling nitric oxide production and getting rid of free radicals Van Kampen et al., 2014. Ginseng also had neuroprotective effect as recorded in various models like stroke He et al., 2012, Alzheimer’s disease Kim et al., 2013, Parkinson's disease Van Kampen et al., 2014, ALS Khabazian et al., 2002, and Huntington's disease Wu et al., 2009. Another study reported that ginseng was effective in the treatment of cardiovascular disease induced by HHcy Kim et al., 2013.
Polygonum multiflorum (pm) extract has been used as an anti-ageing herb in countries of East Asia. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside (THSG) is the main component of Polygonum multiflorum, its structure is similar to resveratrol. Many clinical studies have shown that Polygonum extract reduces progression of hypercholesterolaemia Algohary et al., 2016, cardiac disease, neurosis and diseases commonly related to ageing Lee et al., 2014. Polygonum extract has been reported to have various beneficial effects including antioxidant activity, anti-inflammatory and lipid metabolism regulation Li et al., 2005. Another study revealed that Polygonum has neuroprotective effects against focal ischaemia, monoamine oxidase inhibitor, and improve learning and memory Ling and Xu. Genus of Artemisia (Asteraceae, Anthemideae, Artmisiianae) include about five hundreds of numerous species, spread all over world (geographical areas), and draw more attention due to high economic importance, especially medicinal properties Ben-Nasr et al., 2013. The major bioactive composites of Artemisia judaica (AJ) essential oils (artemisyl-oil, apiperitone-oil, piperitone and trans-ethyl cinnamate), saponins, terpenes, tannins, and flavonoids (apigenin, cirsimaritin, flavonoid glycosides) which exhibit antioxidant, anti-inflammation and antihyperlipidaemic activities El-Massry et al., 2002. Artemisia judaica (AJ) is a permanent plant which spreads widely in the Sinai deserts of Egypt. Reasonably these plants were utilised for decades as, anti-venom, anti-anthelminthic, antidepressant, antiseptic, diuretic, antispasmodic, hypoglycaemic, anti-cancer, antihypolipidaemic, etc. Ben-Nasr et al., 2013. The aim of this study is to compare the possible neuroprotective effects of different herbal cocktail and select the best mixture in HHcy-induced rats’ brain cerebrovascular dysfunction.
Methods
Experiments were performed in weanling Wistar rats, aged 21 days and weighing 40-50 g. They were housed in cages (6/cage) under controlled conditions. Animals were supplied from animal house of National Organization for Drug Control and Research (NODCAR). The animals were fed ad libitum and allowed to adjust to the new environment for two weeks before starting the experiment. The animals were housed at 22 ± 2°C light/dark cycles. Animal use and care for experimental procedure were approved by the Institutional Animal Care and Use Committee (IACUC) of NODCAR.
Extract preparation
Approximately 150 g of Artemisia Judaica leaf were extracted twice with 70% ethanol using a 2 h. reflux extraction, and the extract was concentrated underreduced pressure. The concentrate was filtered, lyophilised, and subsequently stored at 4°C. The yield of the dried extract from starting crude materials was 16.22% (w/w).
Approximately, 100 g of Panax ginseng root was extracted twice with boiling water, filtered, evaporated in a rotary vacuum evaporator, and freeze-dried. The yield of the dried extract from starting crude materials was 21.07% (w/w). Approximately, 1500 g of Polygonum multiflorum root was extracted twice with 70% ethanol using a 2 h. reflux extraction, and the extract was concentrated under reduced pressure. The concentrate was filtered, lyophilised, and subsequently stored at 4°C. The yield of the dried extract from starting crude materials was 13.73% (w/w).
Experimental design
A total of fifty-four male rats (Sprague-Dawley) of 21 days old were utilised in this study and adapted in cages for one week. Rats were randomly divided into nine groups, each comprising six rats. The study was conducted for 30 days after 30 days age.
Homocysteine (Sigma-Aldrich®) (0.03 μmol/kg of b.w.) was administered subcutaneously, twice a day, from the 30th to the 60th day of the life of rats (Scherer et al., 2011). The rats were decapitated 12 h. after the last Homocysteine injection. The brain hippocampus weighed and kept frozen at -80°C until biochemical analysis. Group I: Controls received the same volume of saline solution (0.5 mL/100 kg of b.w.).
Group II: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/kg of b.w. daily for 30 days.
Group III: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/kg of b.w. + Artemisia judaica extract (50 mg/kg b.w. by oral feeding needle with tuberculin syringe) daily for 30 days.
Group IV: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/kg of b.w. + Panax ginseng extract (50 mg/kg per oral by oral feeding needle with tuberculin syringe) daily for 30 days.
Group V: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/g of b.w. + Polygonum multiflorum extract (400 mg/kg per oral by oral feeding needle with tuberculin syringe) daily for 30 days.
Group VI: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/g of b.w. + Artemisia Judaica + Panax ginseng with the same dose of previous group daily for 30 days.
Group VII: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/g of b.w. + Artemisia Judaica + Polygonum multiflorum with the same dose of previous group daily for 30 days.
Group VIII: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/kg of bodyweight + Panax ginseng + Polygonum multiflorum with the same dose of previous group daily for 30 days.
Group IX: served as hyperhomocysteinaemia and received homocysteine 0.03 μmol/kg of b.w. + Artemisia Judaica + Panax ginseng + Polygonum multiflorum with the same dose of the previous group daily for 30 days.
Determination of MDA by HPLC
Preparation of the standard solution
MDA standard was prepared by dissolving 25 μL 1,1,3,3-tetraethoxypropane (TEP) in 100 mL of water to give a 1 mM stock solution. Working standard was prepared by hydrolysis of 1 mL TEP stock solution in 50 mL 1% sulphuric acid and incubation for 2 h at room temperature. The resulting MDA standard of 20 nmol/mL was further diluted with 1% sulphuric acid to yield the final concentration of 1.25 nmol/mL to get the standard for the estimation of total MDA Karatepe, 2004.
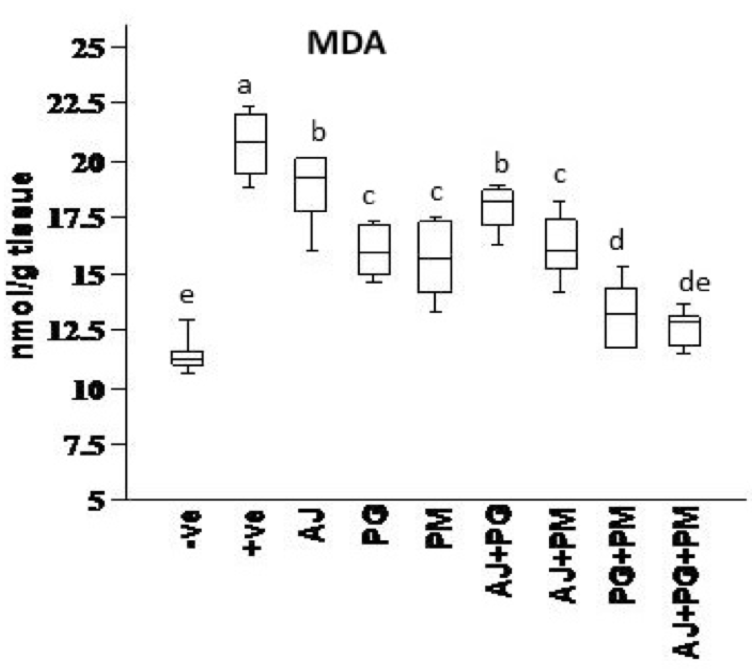
MDA activity was determined after extraction using following protocol: The samples were analysed on an Agilent HP 1100 series HPLC apparatus (USA). The analytical column Supelcosil C18 (5 µm particle and 80 Ao pore size) (250 x 4.6 ID). Mobile phase consists of 30 mmol KH2PO4 and methanol (65%-35%, H3PO4 by pH 4), and the mobile phase at a 1.5 mL/min. flow rate, wavelength 250 nm according to the method of Karatas et al. 2002 Karatas et al., 2002. Data Presented in Figure 3 . The resulting chromatogram identified MDA position and concentration from the sample as compared to that of the standard as previously reported in monoamine content calculation.
Determination of nitrites and nitrates by HPLC
Nitrites and nitrate was determined according to the method of Papadoyannis, Samanidou, and Nitsos 1999 by HPLC Papadoyannis et al., 1999.
Preparation of the standard solution
Sodium nitrite and sodium nitrate used for the reference standard preparation with stock concentration 1 mg/mL. A standard mixture of nitrite and nitrate was used to determine the retention times and separation of the peaks. Nitrite and nitrate concentrations were equal in the mixture solution.
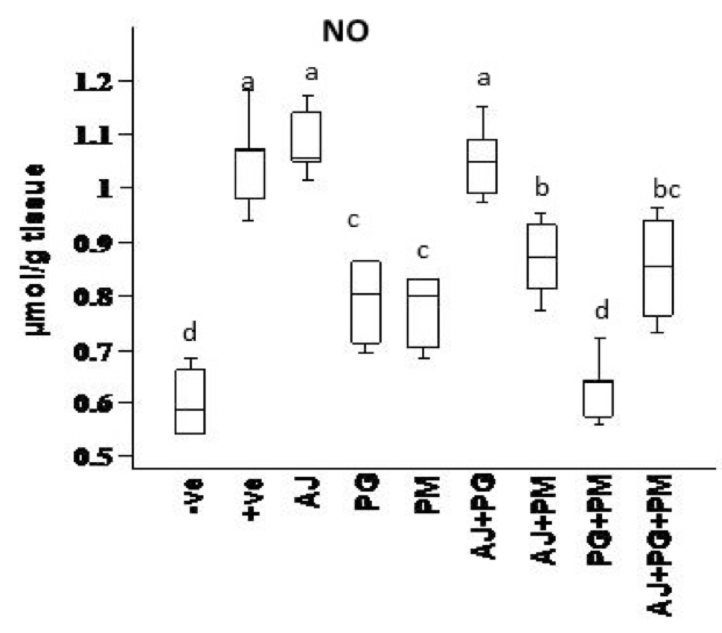
NO was determined after extraction using following protocol: The samples were analysed on an Agilent HP 1100 series HPLC apparatus (USA). The analytical column was anion exchange PRP-X100 Hamilton, 150 x 4.1 mm, 10 μm. The mobile phase was a mixture of 0.1 M NaCl - methanol, at a volume ratio 45:55. The flow rate of 2 mL/min., wavelength adjusted to 230 nm. Data Presented in Figure 4 . The resulting chromatogram identified each of nitrite and nitrate" positions and concentration from the sample as compared to that of the standards.
Determination of Superoxide dismutase (SOD) activity
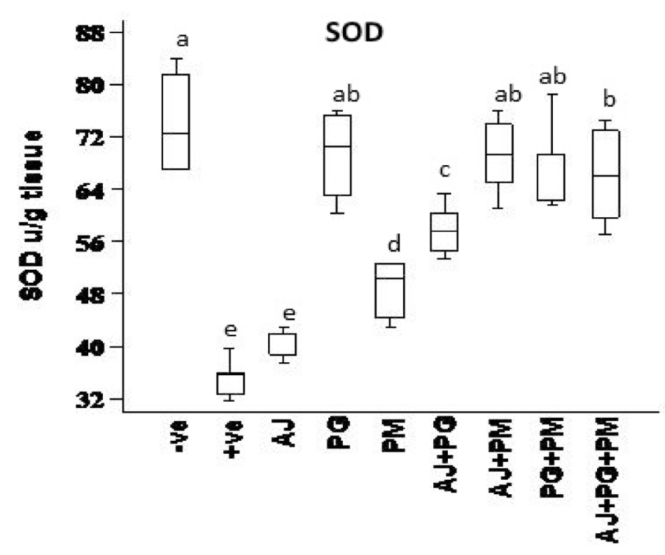
The activity of SOD was determined according to the method of Marklund and Marklund 1974 Marklund and Marklund, 1974. SOD activity was determined after extraction using following protocol: In a spectrophotometer Micro Cuvette, 0.985 of tris-HCL buffer was added to 0.010 mL of Pyrogallol solution and 0.005 mL of double distilled water. Absorbance was measured at 420 nm immediately and after one minute, the resultant A was considered as the experimental blank. The same procedure was carried out by the use of 0.005 mL of the prepared SOD containing solutions (standard) or sample instead of double distilled water.
Determination of Catalase (CAT) activity in brain tissue homogenate
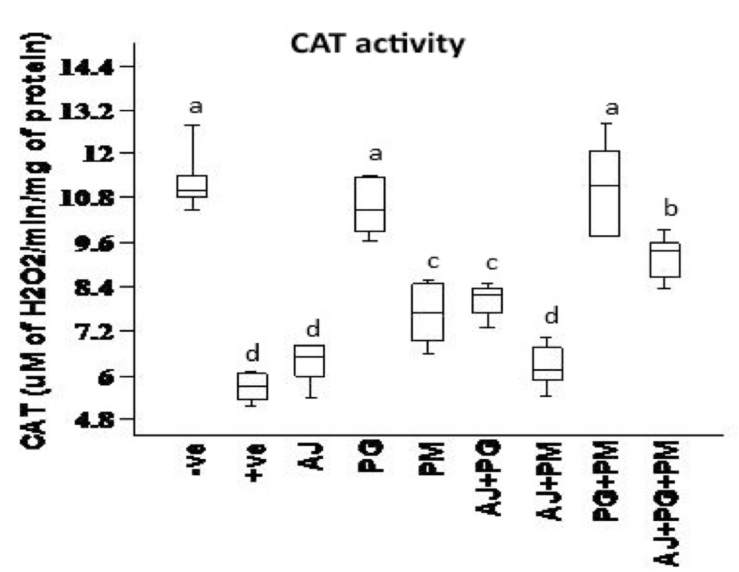
Catalase (CAT) activity was measured by the method of Del Maestro and McDonald 1987. The method is based on the rate of H2O2 degradation by the action of CAT contained in the examined samples followed spectrophotometrically at 230 nm in 5 mM EDTA, 1 M Tris-HCl solution, pH 8.0.
Determination of Acetylcholinesterase (AChE) activity in brain tissue homogenate
The procedure used for the determination of acetylcholinesterase activity in the brain hippocampus samples of rabbit is a modification of Ellman et al. 1961 Ellman et al., 1961 method as described by Gorun et al. 1978 Gorun et al., 1978.
AChE activity was determined after extraction using following protocol: The brain hippocampus tissue samples were weighed and homogenised in a 20-mmol-phosphate buffer, pH 7.6 (5 % w/v). The following reagents were pipettes in a cuvette: 0.14-mL phosphate buffer 20 mmol (pH 7.6), 0.05 mL of 5-mmol acetylthiocholine iodide and 0.01 mL of tissue homogenate or serum. After 10 min. of incubation at 38°C, the reaction was stopped with 1.8 mL of DTNB – phosphate ethanol reagent. The colour was read immediately at 412 nm using Shimadzu spectrophotometer UV –1601. Omitting the enzyme from the incubation mixture made the control samples. After addition of the colour reagent, appropriate amount of tissue homogenates or serum was added to the control. The cholinesterase activity was determined as µmol SH from a standard curve.
Determination of IL-6 in the brain
IL-6 levels in the rat brain were estimated using a rat-specific immunoassay kit (Rat IL-6 ELISA) from Glory Science (Del Rio, Texas, USA) according to the manufacturer's protocol. The intensity of the coloured product was directly proportional to the concentration of rat IL-6, as evaluated using a micro plate reader (Biotech ELx800; Biotech Instruments) set at 450 nm. The sample concentration was determined against a standard curve and is expressed in nanograms of IL-6 per gram of brain tissue.
Determination of BDNF in the brain
BDNF levels were estimated using a rat-specific immunoassay kit (Rat BDNF ELISA) from Glory Science, according to the manufacturer's protocol. The intensity of the coloured product was directly proportional to the concentration of rat BDNF, as determined using a micro plate reader (Biotech ELx800) set at 450 nm. The sample concentration was determined against a standard curve and is expressed in nanograms.
DNA Comet Assay
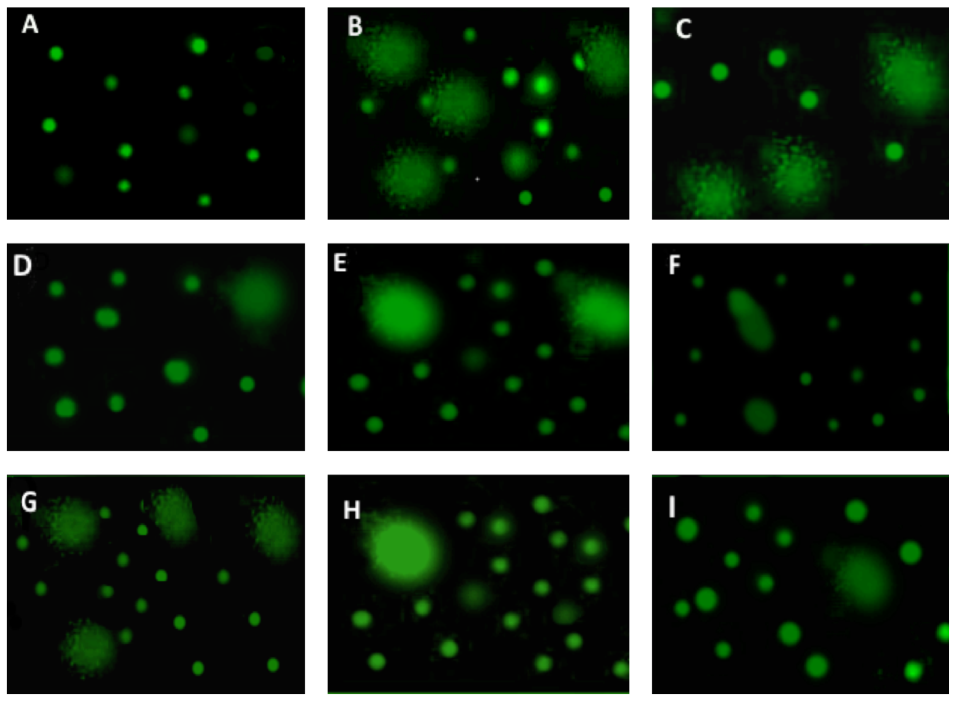
Comet assay of DNA was estimated according to the classic alkaline single-cell electrophoresis protocol Xu et al., 2013. Samples were stained with SYBR Green I (Sigma-Aldrich, St Louis, MO) and analysed by Comet Score 1.5 software. Percent of DNA in comet tails was considered as the marker of genotoxic effect.
Statistical analysis
The values were expressed as the mean ± SE for the 6 male rats in each group. Differences between groups were assessed by one way analysis of variance (ANOVA) using SAS (2004) software for Windows (version 13.0). Statistical analysis of the obtained data was performed using the general linear model (GLM). Significant differences among means were evaluated using Duncan’s (1955) Duncan, 1955. Multiple Range Test.
The following linear model was applied: Yij = μ + αi + ξij, Yij = Observation measured, Μ = Overall mean, αi = Effect of treatment. ξij = Experimental error assumed to be randomly distributed ( σ2 = 0 ).
Results
The effect of some herbal extracts on nitric oxide (ACHE), catalase (CAT), malondialdehyde (MDA) and superoxide dismutase (SOD) in hyperhomocysteinaemic rats
Data presented in Figure 1 , Figure 2 , Figure 3 and Figure 4 records the effect of homocysteine oral administration on antioxidant parameter (CAT, MDA, NO and SOD) of hippocampus brain area at the end of experiment. Homocysteine induces a significant decrease (p<0.05) in the hippocampus CAT and SOD and significant increase (p<0.05) of NO and MDA compared to control group. On the other hand, PG, PM, AJ+PG, PG+PM and AJ+PG+PM showed significant differences (p<0.05) against homocysteine group and rounded than the control group. Also, AJ+PM showed ameliorative response and nearly rounded to control for CAT, NO, MDA and SOD compared with homocysteine group but AJ only didn’t show any improvement than homocysteine group.
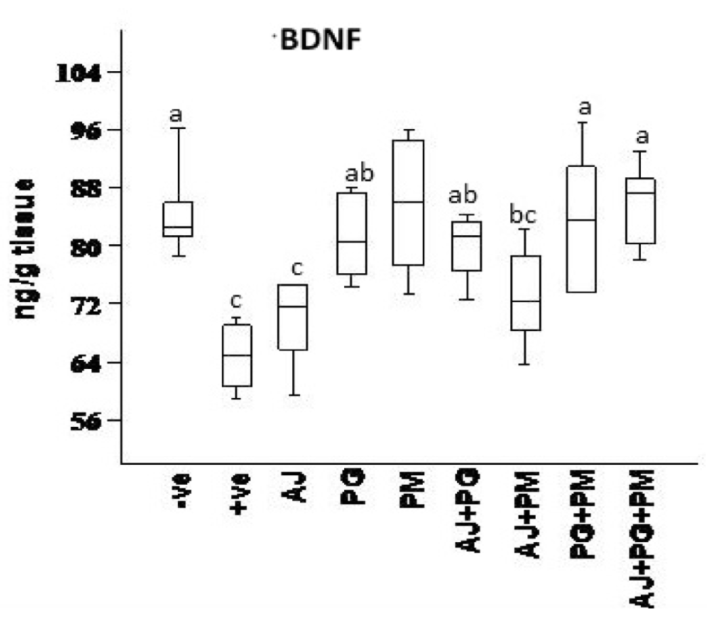
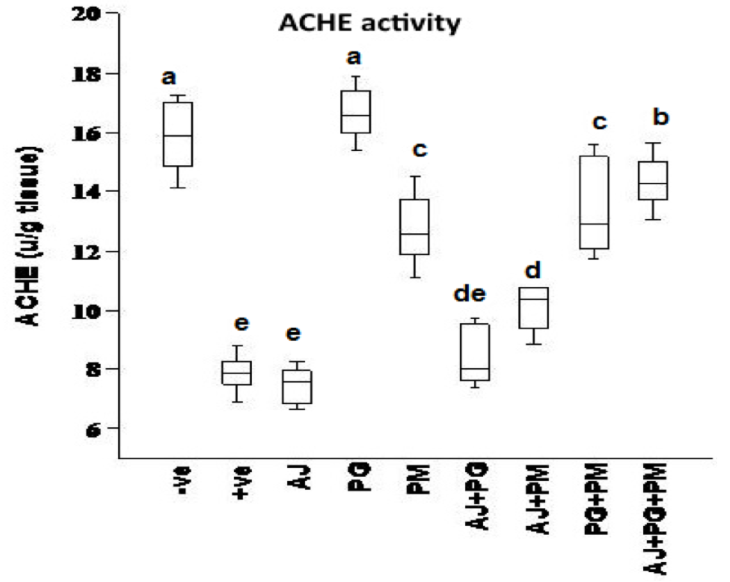
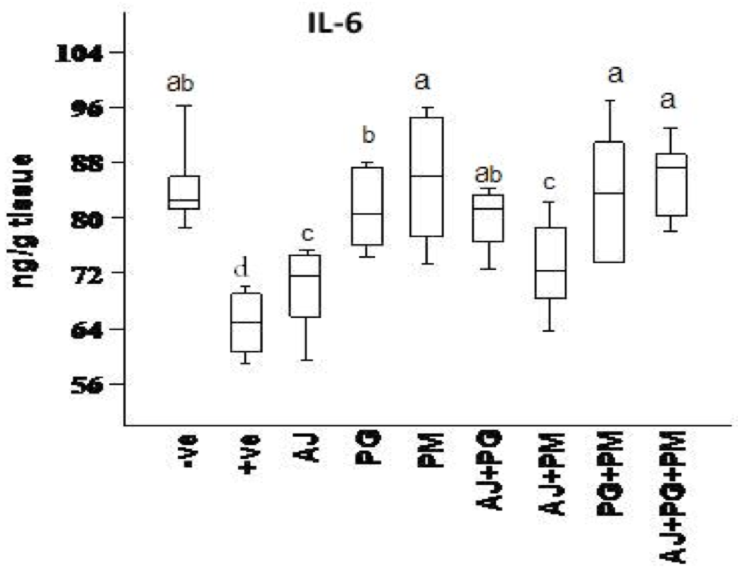
The effect of some herbal extracts on (ACHE), (IL6) and (BDNF) in hyperhomocysteinaemic rats
As showed in Figure 5 , Figure 6 , and Figure 7 , records the effect of homocysteine oral administration on IL-6, BDNF and SOD of hippocampus brain area at the end of experiment. Homocysteine caused elevation in inflammatory markers (IL-6), and AChE of hippocampus brain area. Homocysteine also induces a significant decrease (p<0.05) in the hippocampus neurotrophic factor (BDNF) as compared to control group. On the other hand, all treated group except AJ only showed significant (p<0.05) ameliorative effect of SOD, IL-6, and BDNF against homocysteine group and rounded than the control group, but AJ only didn’t show any improvement than homocysteine group at the same parameters.
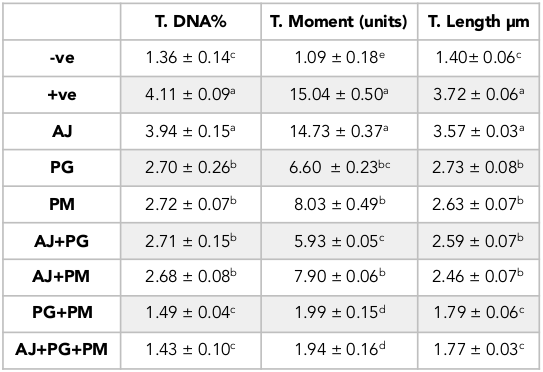
Concerning the brain genotoxic potential of homocysteine using the comet essay, there was a significant increase in the tail length of DNA, tail intensity (DNA%) and tail moment in the homocysteine-treated rats compared to the control. On the other hand, the PG and PM treated groups significantly decreased DNA tail length, intensity and moment as compared to the homocysteine-treated rats, while the AJ treated groups showed no pronounced effect ( Table 1 and Figure 8 ).
Discussion
Stroke, one of the most risks of severe disability and death, data from numerous studies concluded that stroke and ischaemic disease accompanied by elevated homocysteine level Boysen et al., 2003.
Hyperhomocysteinaemia caused endothelial dysfunction by the low density lipoproteins direct oxidation, its cytoplasmic oxidation of compounds like homocysteine, mixed disulphides and homocysteine thiolactone, which lead to the release of free radicals as reactive oxidative species ROS (hydrogen peroxide, superoxide anion and hydroxide radical) and acceleration of fibrin and collagen accumulation in endothelia Plazar and Jurdana, 2012. In present study, homocysteine caused elevation in oxidative stress (MDA and NO), and inflammatory markers (IL-6) of hippocampus brain area. Homocysteine also induces a significant decrease (p<0.05) in the hippocampus CAT, SOD, neurotrophic factor (BDNF) and significant increase (p<0.05) of AChE compared to control group. Another study suggested that HHcy caused elevation of oxidative stress markers, impaired endothelium vasorelaxation and suppression endothelial nitric oxide synthase Zhou et al., 2005.
On the other hand, the recent study extends to evaluate the neuroprotective effects of some herbal extracts. The protective effects of PG, PM, AJ and its mixtures against hyperhomocysteinaemia are investigated, PG, PM, AJ+PG, PG +PM and AJ+PG+PM showed notable differences against homocysteine group and rounded than the control group. Also, AJ+PM showed ameliorative response and nearly rounded to control for SOD and AChE compared with homocysteine group, but AJ only didn’t show any improvement as compared to homocysteine group, all treated group also showed significant differences of MDA against homocysteine group and rounded than the control group. Also, all treated group except AJ only showed vital effects of NO, IL-6, and BDNF against homocysteine group and rounded than the control group, but AJ only didn’t show any improvement than homocysteine group at the same parameters.
Oral administration of PG could modulate the toxic effect of homocysteine. Several studies suggested that the antioxidant and vasorelaxation effect of ginseng is the key role in its neuroprotective effect. Bioactive components of PG (ginsenosides) have high antioxidant activity and blocking the oxidation of lipids Zhou et al., 2005. These phenolic compounds such as flavonoids, phenolic acids, diterpenes, saponins, and tannins could reduce cell damage induced by oxidative stress Algohary et al., 2016. In recent study, PM extract treatment alone also showed a protective effect against hyperhomocysteinaemia. This is in agreement to several studies that suggested PM had protective effect against ageing and other diseases commonly related to ageing, as stroke, ischaemia, hyperlipidaemia, coronary heart disease, neurosis Lee et al., 2014. This may be due to Stilbene glycoside, the bioactive compound of PM. It is similar to resveratrol, exhibits many pharmacological properties, including antioxidant, anti-inflammatory, and lipid regulator Li et al., 2005. Stilbene, Resveratrol, and other polyphenolic compounds may also increase nitric oxide bioavailability, thereby supressing the progress of endothelial dysfunction; reducing blood viscosity, improving insulin sensitivity, counteracting platelet hyperactivity, suppressing platelet adhesion to fibrinogen-coated surfaces Malinowska and Olas, 2011. Stilbene glycoside also caused significant improvement in learning and memory by reduction of MDA, and monoamine oxidase in the cerebral cortex. Another study revealed that stilbene glycoside inhibits microglial inflammation by releasing of proinflammatory markers as TNF-α, IL-1β, IL-6, and NO Ling and Xu, 2016.
The co-administration of PG and PM in the recent study enhanced there vital effects, as compared to its individual treatment.
Conclusion
In conclusion, the present study suggests that herbal cocktail (mixture of PG 50 mg/kg b. wt. and PM 400 mg/kg b. wt. may reduce the toxic effects of Hcys by decreasing oxidative stress, releasing pro-inflammatory mediators, increasing NO bioavailability and inhibiting the progress of endothelial dysfunction. Therefore, may be potentially useful in the prevention of hyperhomocysteinemia and other related cerebrovascular diseases.
Abbreviations
AChE: Acetylcholinesterase
AJ: Artemisia Judaica extract
ANOVA: Analysis of variance
BBB: blood-brain barrier
BDNF: Brain-derived neurotrophic factor
CAT: Catalase
GLM: general linear model
HHcy: Hyperhomocysteinaemia
IACUC: Institutional Animal Care and Use Committee
IL-6: Interleukin - 6
MDA: Malondialdehyde
NO: nitric oxide
NODCAR: National Organization for Drug Control and Research
PG: Panax ginseng extract
PM: Polygonum multiflorum extract
SOD: Superoxide dismutase
TEP: 1,1,3,3-tetraethoxypropane
THSG: 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside
Author contribution
Dr. A.M. Algohary and Dr. R. Al-Baradie contributed in paper with phyto analysis and extraction (40%). Dr. O.A. Ahmed-Farid and Dr. A.M. Abd-Elrazek contributed in paper with biological modeling and evaluation in animal of all extraction (60%).
References
-
A.
Algohary,
R.A.
Baradie,
O.
Farid,
A.
Elrazek,
A.A.
Sulaiman.
Developing a Herbal Cocktail for prevention of Stroke and cerebrovascular diseases. Journal of Biomedical and Pharmaceutical Research.
2016;
5
.
-
H.
Ben-Nasr,
M.A.B.
Abderrahim,
M.
Salama,
K.
Ksouda,
K.-M.
Zeghal.
Potential Phytotherapy use of Artemisia Plants: Insight for Anti-Hypertension. 2013
.
-
G.
Boysen,
T.
Brander,
H.
Christensen,
R.
Gideon,
T.
Truelsen.
Homocysteine and risk of recurrent stroke. stroke.
2003;
34
:
1258-1261
.
-
D.B.
Duncan.
Multiple range and multiple F tests. Biometrics.
1955;
11
:
1-42
.
-
K.F.
El-Massry,
A.H.
El-Ghorab,
A.
Farouk.
Antioxidant activity and volatile components of Egyptian Artemisia judaica L. Food Chemistry.
2002;
79
:
331-336
.
-
G.L.
Ellman,
K.D.
Courtney,
V.
Andres,
R.M.
Featherstone.
A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology.
1961;
7
:
88-95
.
-
V.
Gorun,
I.
Proinov,
V.
Băltescu,
G.
Balaban,
O.
Bârzu.
Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Analytical biochemistry.
1978;
86
:
324-326
.
-
B.
He,
P.
Chen,
J.
Yang,
Y.
Yun,
X.
Zhang,
R.
Yang,
Z.
Shen.
Neuroprotective effect of 20 (R)-ginsenoside Rg 3 against transient focal cerebral ischemia in rats. Neuroscience letters.
2012;
526
:
106-111
.
-
F.
Karatas,
M.
Karatepe,
A.
Baysar.
Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Analytical biochemistry.
2002;
311
:
76-79
.
-
M.
Karatepe.
Simultaneous determination of ascorbic acid and free malondialdehyde in human serum by HPLC-UV. Lc Gc North America.
2004;
22
:
362-365
.
-
I.
Khabazian,
J.
Bains,
D.
Williams,
J.
Cheung,
J.
Wilson,
B.
Pasqualotto,
S.
Pelech,
R.
Andersen,
Y.T.
Wang,
L.
Liu.
Isolation of various forms of sterol β-d-glucoside from the seed of Cycas circinalis: neurotoxicity and implications for ALS-parkinsonism dementia complex. Journal of neurochemistry.
2002;
82
:
516-528
.
-
E.-J.
Kim,
I.-H.
Jung,
T.K.
Van Le,
J.-J.
Jeong,
N.-J.
Kim,
D.-H.
Kim.
Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. Journal of ethnopharmacology.
2013;
146
:
294-299
.
-
M.
Kumar,
N.
Tyagi,
K.S.
Moshal,
U.
Sen,
S.
Kundu,
P.K.
Mishra,
S.
Givvimani,
S.C.
Tyagi.
Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochemistry international.
2008;
53
:
214-219
.
-
S.V.
Lee,
K.H.
Choi,
Y.W.
Choi,
J.W.
Hong,
J.U.
Baek,
B.T.
Choi,
H.K.
Shin.
Hexane extracts of Polygonum multiflorum improve tissue and functional outcome following focal cerebral ischemia in mice. Molecular medicine reports.
2014;
9
:
1415-1421
.
-
X.
Li,
K.
Matsumoto,
Y.
Murakami,
Y.
Tezuka,
Y.
Wu,
S.
Kadota.
Neuroprotective effects of Polygonum multiflorum on nigrostriatal dopaminergic degeneration induced by paraquat and maneb in mice. Pharmacology Biochemistry and Behavior.
2005;
82
:
345-352
.
-
S.
Ling,
J.-W.
Xu.
Biological Activities of 2, 3, 5, 4'-Tetrahydroxystilbene-2-O-β-D-glucoside in Antiaging and Antiaging-related Disease Treatments. 2016
.
-
J.
Malinowska,
B.
Olas.
Response of blood platelets to resveratrol during a model of hyperhomocysteinemia. Platelets.
2011;
22
:
277-283
.
-
S.
Marklund,
G.
Marklund.
Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European journal of biochemistry.
1974;
47
:
469-474
.
-
I.
Papadoyannis,
V.
Samanidou,
C.C.
Nitsos.
Simultaneous determination of nitrite and nitrate in drinking water and human serum by high performance anion-exchange chromatography and UV detection. Journal of liquid chromatography & related technologies.
1999;
22
:
2023-2041
.
-
N.
Plazar,
M.
Jurdana.
Hyperhomocysteinemia: relation to cardiovascular disease and venous thromboembolism. INTECH Open Access Publisher.
2012
.
-
G.
Sainani,
P.
Talwalkar,
R.
Wadia,
A.
Keshvani.
Hyperhomocysteinemia and its implications in atherosclerosis the Indian Scenario. Medicine Update.
2007;
17
:
11-20
.
-
J.M.
Van Kampen,
D.B.
Baranowski,
C.A.
Shaw,
D.G.
Kay.
Panax ginseng is neuroprotective in a novel progressive model of Parkinson's disease. Experimental gerontology.
2014;
50
:
95-105
.
-
J.
Wu,
H.K.
Jeong,
S.E.
Bulin,
S.W.
Kwon,
J.H.
Park,
I.
Bezprozvanny.
Ginsenosides protect striatal neurons in a cellular model of Huntington's disease. Journal of neuroscience research.
2009;
87
:
1904-1912
.
-
X.-R.
Xu,
J.-Q.
Zhu,
T.
Ye,
C.-L.
Wang,
Y.-F.
Zhu,
H.-U.
Dahms,
F.
Jin,
W.-X.
Yang.
Improvement of single-cell gel electrophoresis (SCGE) alkaline comet assay. Aquatic Biology.
2013;
18
:
293-295
.
-
W.
Zhou,
H.
Chai,
P.H.
Lin,
A.B.
Lumsden,
Q.
Yao,
C.
Chen.
Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg.
2005;
41
:
861-868
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 12 (2016)
Page No.: 1045-1061
Published on: 2016-12-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6592 times
- Download PDF downloaded - 2773 times
- View Article downloaded - 19 times
 Biomedpress
Biomedpress