A comparison of umbilical cord blood-derived endothelial progenitor cell transplantation and mononuclear cell transplantation for the treatment of acute hindlimb ischemia in a murine model
Abstract
Introduction: Acute lower limb ischemia is a common peripheral artery disease whose treatment presents many difficulties. Stem cell transplantation is considered a novel and promising method of treating this disease. Umbilical cord blood (UCB) is rich in stem cells, including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs). However, historically, banked umbilical cord blood has been used mainly to treat blood-related diseases. Therefore, this study compared the efficacy of umbilical cord blood-derived mononuclear cells (UCB-MNCs) with EPC transplantation for the treatment of acute hindlimb ischemia (ALI) in mouse models.
Methods: MNCs were isolated from UCB by Ficoll gradient centrifugation, after which the EPCs were sorted based on CD34+ and CD133+ markers and cultured according to a previously published protocol. To induce ALI, mice were immuno-suppressed using busulfan (BU) and cyclophosphamide (CY), after which the femoral arteries were burned. Induction of ALI in the immune suppressed mice was confirmed by the grade of tissue damage, pedal frequency in water, tissue edema, changes in histology, total white blood cell count, and white blood cell composition. Model mice were injected with a dose of MNCs or EPCs and un-treated control mice were injected with phosphate buffered saline. The efficiency of treatment was evaluated by comparing the grade of tissue damage between the three groups of mice.
Results: Mice aged 6–12 months were suitable for ALI, with 100% of mice exhibiting ischemia from grade I 10%, grade III 50%, grade IV 40%. For all ALI mice, a gradual increase in pedal frequency in water, increased tissue edema, necrosis of muscle tissue, and (Andaz, 1993 #6)loss of hindlimb function were observed after 20 days. Transplanted MNCs and EPCs significantly improved hindlimb ischemia compared with control treatment. Moreover, EPC transplantation significantly improved hindlimb ischemia compared with MNC transplantation. Following EPC and MNC transplantation, 44.44% and 33.33% of the mice recovered fully (grade 0), respectively. Specifically, all recovered mice exhibited hindlimb activities similar to those of normal mice
Introduction
Ischemia is conditionally caused by limited blood supply to tissues, which results in a shortage of oxygen and the nutrients needed for cellular activities. The main cause of ischemia is damage to blood vessels. Acute hindlimb ischemia (ALI) is a medical condition caused by a sudden lack of blood flow to hindlimbs Walker, 2009. ALI is caused by either an embolism or thrombosis. Thus, ALI can be caused by peripheral vascular disease, trauma, excess fat, amniotic fluid or a tumor Jaffery et al., 2011. Prolonged ALI or delayed treatment can result in morbidity, amputation and/or death. Therefore, ALI has been researched and treated for many years.
At present, almost all therapies for treating ALI are based on thrombolysis. Thrombosis can be removed by various methods, including the use of streptokinase, tissue plasminogen activator, urokinase and anistreplase Andaz et al., 1993, Callum and Bradbury, 2000, Wardlaw and Warlow, 1992. Other methods of clot lysis use either saline jets or ultrasonic waves. Saline jets can dislodge the clot by the Bernoulli effect, whereas ultrasonic waves can physically fragment the thrombus Lyden, 2010. Alternatively, a “bypass” can be created around the clot by inserting a graft Corfield et al., 2013.
In recent years, stem cell therapy has been considered as another method for treating ALI. Compared with the methods for treating ALI described above, stem cell therapy promises many advantages. Indeed, stem cells can create new blood vessels that supply oxygen and nutrients to the ischemic area. Stem cells can be obtained from two main sources, namely embryos and adult tissues. The use of embryonic stem cells has limitations because of teratoma formation and ethical dilemmas, whereas adult stem cells require invasive techniques for their collection.
Human umbilical cord blood (UBC) is a rich source of stem cells. There are at least three kinds of stem cells in UBC, including mesenchymal stem cells, endothelial progenitor cells and hematopoietic stem cells. UCB-derived stem cells have been used in preclinical trials and clinical applications for many years. To date, more than 25,000 allogeneic cord blood transplantations have been performed worldwide since the first cord blood transplantation in 1998 Butler and Menitove, 2011. More than 780,000 cord blood units are stored in over 130 private cord blood banks worldwide, and over 400,000 units in more than 100 quality-controlled public cord blood banks Butler and Menitove, 2011. Early uses of UCB-derived stem cells included the preparation of a mononuclear cell fraction by gradient centrifugation to remove red blood cells and white blood cells Fuss et al., 2009, Jaatinen and Laine, 2007. Recently, automated systems for isolating UCB-derived stem cells for clinical applications have been developed; however, they are mainly used for mononuclear cell isolation or mononuclear cell enrichment. Three main types of stem cells, hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs), are included in the mononuclear cell fraction. In fact, HSCs Sousa et al., 2011, Notta et al., 2011, Chularojmontri and Wattanapitayakul, 2009, Delalat et al., 2009, Kent et al., 2009, MSCs Li and Cai, 2012, and EPCs Lin et al., 2011, Mead et al., 2008, Duan et al., 2008, Song et al., 2010, Phuc et al., 2012 have all been successfully isolated from the mononuclear cell fraction.
Both the MNC fraction and EPCs were used for the treatment of ischemic hindlimb a decade ago Yang et al., 2004, Liu et al., 2005, Murohara et al., 2000, Finney et al., 2006. EPCs induced with cytokines, such as stem cell factor, FMSlike tyrosine kinase 3 ligand, interleukin-3, and basic fibroblast growth factor O et al., 2011, and transgenic EPCs expressing VEGF Yu et al., 2009, significantly improved the blood flow as well as neoangiogenesis in hindlimb ischemia. Treatment with UCB MNCs has also shown an improvement in ischemia Henning et al., 2012, Pimentel-Coelho et al.,2012. UCB MNCs injected in combination with the five growth factors, Flt-3L, EGF, TPO, FGF and IGF-1, prevented limb loss and augmented blood perfusion, capillary density, vascular maturation and angiogenic cytokines in the affected tissues Kim et al., 2011. Cho et al. (2006) injected UCB MNCs using a fibrin matrix in combination with delivery of bFGF, and found that capillaries and arterioles were significantly increased compared with therapy alone as well as control Cho et al., 2006.
MNCs and EPCs present advantages and disadvantages regarding their isolation. Currently, however, the efficacy of hindlimb ischemia treatment with MNC or EPC transplantation has not been compared. Therefore, in this study we compared the efficacy of MNC and EPC transplantation for treating hindlimb ischemia in murine models, to evaluate the suitability of allogeneic transplantation for hindlimb ischemia treatment.
Materials – Methods
Isolation of the mononuclear cell (MNC) fraction from human umbilical cord blood
Umbilical cord blood was collected from consenting donors screened for HBV, HCV, and HIV at Hung Vuong hospital, Ho Chi Minh city, Vietnam. All procedures and manipulations were approved by our Institutional Ethical Committee (Laboratory of Stem cell Research and Application, University of Science, Vietnam National University, Ho Chi Minh City, Vietnam) and the Hospital Ethical Committee (Hung Vuong Hospital, Ho Chi Minh City, Vietnam). The bloods were transferred to the laboratory in a cool box within 3 h. The mononuclear cell fraction was isolated by gradient density centrifugation using Ficoll-Histopaque 1.077 (GE Healthcare) according to the manufacturer’s guidelines. Finally, the cell pellets were washed three times with PBS. Cell viability was determined by flow cytometric analysis (FACSCalibur, BD Bioscience; USA) of cells stained with 7-AAD (7-amino actinomycin; BD Bioscience, USA). The presence of hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) was determined by flow cytometry.
Isolation of endothelial progenitor cells from the mononuclear cell fractions
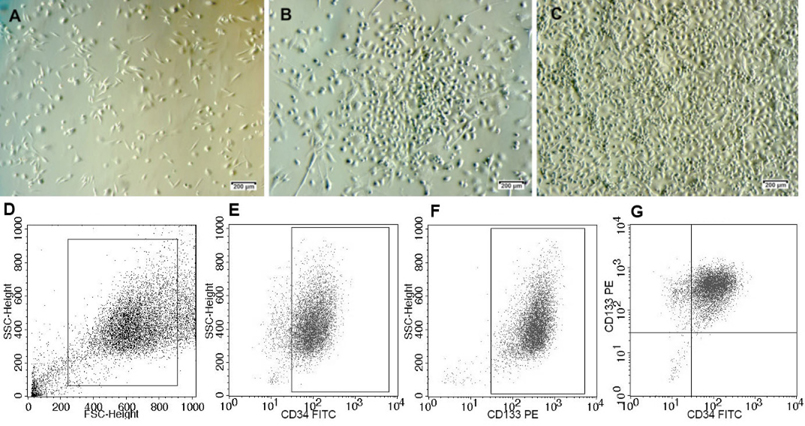
A Cell Sorter FACSJazz System (BD Biosciences, Franklin Lakes, NJ) was used to sort CD34+ CD133+ EPCs from the MNC fraction. MNCs were washed twice in phosphatebuffered saline containing 1% bovine serum albumin (Sigma-Aldrich, St Louis, MO). Fc receptors were blocked by incubation with immunoglobulin G (Santa Cruz Biotechnology, CA) on ice for 15 minutes. MNCs were double-stained with anti-CD34-FITC and anti-CD133-PE monoclonal antibodies (BD Pharmingen, USA) for 30 minutes in the dark. After washing, the MNCs were analyzed and sorted into EPCs expressing CD34+ and CD133+ markers using the cell sorter.
Isolated EPCs were cultured in EBM-2 medium supplemented with EGM-2 (Gibco, Invitrogen) and 1% antibioticmycotic (Sigma-Aldrich, St Louis, MO, USA). This population was considered as EPCs. The EPC population was expanded for a further 18–30 days in the same flask, with the addition of fresh medium every 3 days, before harvesting for flow cytometric analysis. All flasks and dishes were incubated at 37°C in 5% CO2.
Identification of stem cells in the mononuclear cell fractions and EPCs
The presence of stem cells in the MNC fractions, including MSCs expressing CD44+ and CD90+, EPCs expressing CD34+ and CD133+, and HSCs expressing CD34+ and CD45+, was analyzed by flow cytometry on a FACSCalibur system (BD Biosciences, Franklin Lakes, NJ). Briefly, cells were washed twice in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA). Fc receptors were blocked by incubation with immunoglobulin G (Santa Cruz Biotechnology, CA) on ice for 15 minutes. Cells were double-stained with anti-CD34-FITC and anti-CD45-APC monoclonal antibodies for HSCs, anti-CD44-PE and anti-CD90-FITC for MSCs, or anti-CD34-FITC and anti-CD133-PE for EPCs (all monoclonal antibodies were purchased from BD Biosciences, Franklin Lakes, NJ) at 4°C for 30 minutes. After washing, the cells were analyzed by flow cytometry using CellQuest Pro software (BD Biosciences, Franklin Lakes, NJ), with 10,000 events collected.
Immune-suppressed mouse models
All procedures on animals were approved by the Animal Welfare Committee of the Stem Cell Research and Application Laboratory, University of Science, VNU-HCM, VN. Mice were kept in individual ventilated cages with a HEPA filter (Techniplast, Italy). To carry out the xenogenic transplantation of the mononuclear cell fraction and EPCs into mice, the mouse immune system was suppressed using busulfan (BU) and cyclophosphamide (CY). To determine the optimal combined dose of BU and CY, the following three doses were used: 250 mg/kg of CY and 0 mg/kg of BU (N1); 20 mg/kg of BU and 50 of mg/kg of CY divided into 4 continuous injections over 4 days (N2); and 40 mg/kg of BU and 50 mg/kg of CY divided into 4 continuous injections over 4 days (N3). Control mice were injected with PBS. There were 9 mice in each group.
The efficiency of immune suppression was evaluated by the percentage of viable mice (%), and decrease in total white blood cells on day 0 (before injection), day 7, day 14 and day 21 following injection with BU and CY (or PBS in the control group). The optimal dose was used to determine the maintenance dose of CY (25 mg/kg). One dose of CY (25 mg/kg) was used to maintain the immune suppression according to three different modes, including one injection for 2 days (N2a), one injection for 3 days (N2b), and one injection for 4 days (N2c). The efficiency of maintaining immune suppression was also evaluated by total white blood cell counts. The optimal combined dose of BU and CY and the optimal maintenance mode were used to immune suppress the mice before establishing the acute ischemic hindlimb models.
Establishment of hindlimb acute ischemia model mice
Hindlimb ischemia model mice were established according to published protocols Goto et al., 2006 using immunesuppressed mice. All procedures on animals were approved by the Animal Welfare Committee of the Stem Cell Research and Application Laboratory, University of Science, VNUHCM, VN. In this experiment, we investigated the effects of mouse age on the efficiency of model establishment. The mice used to establish the ischemic hindlimb models were aged 3–5 months or 6–12 months. Briefly, the mice were anesthetized using 7.5mg/kg zoletil and the hair was then removed from the hindlimb. Using fine forceps and surgical scissors, an incision approximately 1 cm long was made in the skin. Next, the femoral artery was dissected and separated from the femoral vein and nerve at the proximal location near the groin. After dissection, the mice were placed onto the electrode plate of an electronic cutting machine (ESU-X, Geister, Germany). An electronic knife was used to burn two sites in the femoral arteries. The first site was at the proximal end of the femoral artery. The femoral artery was separated from the femoral vein at the distal location close to the knee. The second site was at close to the knee.
Ischemic mouse models and evaluation of treatment efficiency
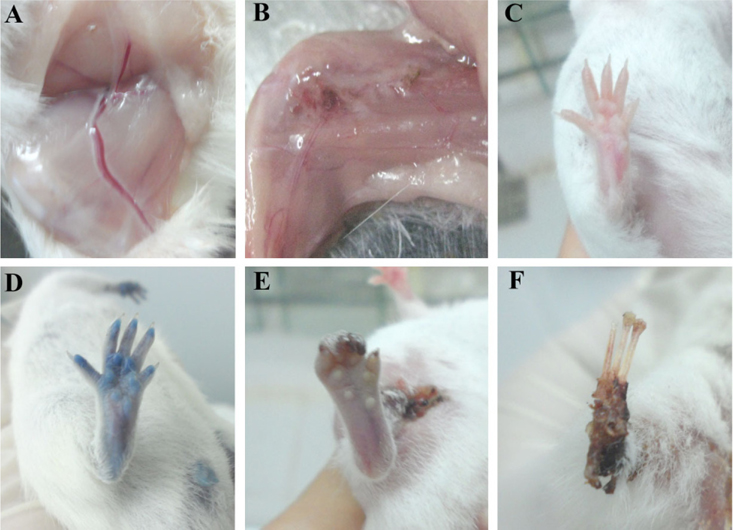
First, the degree of ischemic damage was evaluated according to the guidelines of Goto et al. (2006) Goto et al., 2006. The degree of ischemic damage was recorded and classified as follows: grade 0, an absence of necrosis; grade I, necrosis limited to the toes; grade II, necrosis extending to the dorsum pedis; grade III, necrosis extending to the crus; and grade IV, necrosis extending to the thigh. To confirm that the blood flow could not enter the hindlimb, 0.4% trypan blue was injected into the portal vein.
Second, the pedal response of normal and grade I–IV mice in water was evaluated by counting the pedal frequency of the hindlimbs over a 10-s period.
Third, tissue edema was evaluated one week after transplantation for grade II, III, IV and normal mice, and 72 h after ischemia induction for untreated mice. The ischemic muscle (distal thigh and calf muscle) was isolated and the tissue samples were weighed and then placed in a drying oven at 55°C until a constant weight was observed (usually 36–48 h). The degree of muscle edema was quantitated using the wetto-dry weight ratio (W/D). The W/D was compared between normal mice and experimental groups.
Finally, the histology of limb tissues was analyzed after 4 weeks. Grade I (untreated) mice were evaluated individually three times, including after 72 and 120 h, because of leg loss after 120 h. Muscles were dissected out, rinsed in PBS for 1 h and then dehydrated. Samples were cut at cross sections of 2 m thicknesses and then stained with hematoxylin-eosin (HE). Stained slides were examined by microscopy at 20× magnification (Carl-Zeiss, Germany).
Stem cell transplantation
Acute immune suppressed hindlimb ischemic mice were divided into three groups, with 30 mice in the un-treated group, 30 mice in the umbilical cord blood-derived mononuclear cell transplantation (MNC) group, and 18 mice in the endothelial progenitor cell transplantation (EPC) group. In the un-treated group, the mice were injected with PBS and used as negative controls. In the second group (MNC), the mice were injected with a dose of 106 UCB-MNCs into the ischemic region. In the third group (EPC), the mice were injected with a dose of 106 UCB-EPCs. All mice were followed up for 20 days to evaluate the effects of the grafted stem cells.
After burning the femoral arteries of the immunesuppressed mice for 3–4 hours, all model mice were transplanted with MNCs, EPCs or PBS. The cells were directly injected into the muscle at the burn sites. Treatment efficiency was evaluated by the recovery of ischemic tissue damage in the ischemic hindlimbs. To evaluate the functions of the recovered ischemic hindlimbs, the grade 0 mice in the three groups of normal, MNC and EPC mice were evaluated for tissue edema, pedal frequency and histology on day 21.
Statistical analysis
Data are presented as the mean ± standard error of the mean. Comparisons were made using ANOVA and Student’s t-test. A value of P < 0.05 was considered significant.
Results
Isolation of MNCs and EPCs
Ten UCB samples were used in this research. MNCs were successfully isolated from all samples (10/10; 100%) by Ficoll gradient centrifugation. Analysis of all MNC samples showed that HSCs, EPCs and MSCs accounted for 0.13 ± 0.03%, 1.27 ± 0.11% and 4.73 ± 0.27% of MNCs, respectively (P < 0.05). Five MNC samples were subsequently used for xenotransplantation in murine models, and five samples were used for EPC isolation.
Sorted EPCs were expanded for 2–3 passages. They exhibited the particular shape, similar to our previous published study Phuc et al., 2012 and the expression of surface markers was confirmed before transplantation. It was found that 90.32 ± 0.33% of the cells exhibited the CD34+CD133+ phenotype ( Figure 1 ).
Suppression of the immune system
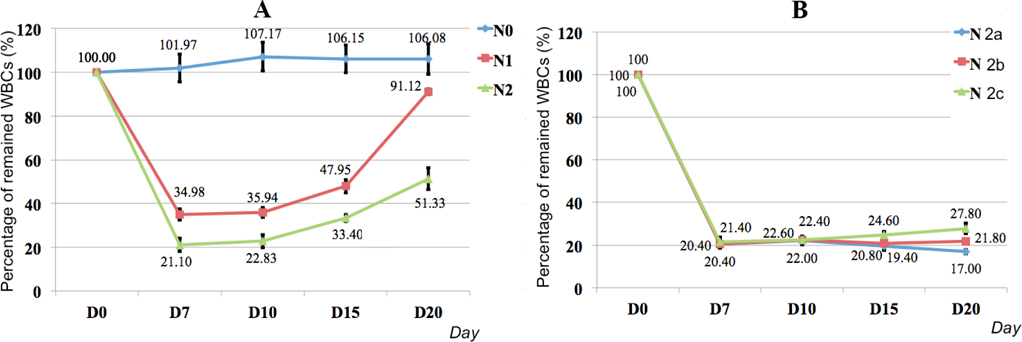
We first evaluated the viability of the mice in the three groups (N1, N2 and N3). In the N3 group, 60% of the mice died 7 days after injection while 100% of the mice remained alive in groups N1 and N2. Therefore, we evaluated the mice in groups N1 and N2 only.
Figure 2 shows that the WBCs gradually decreased from day 0 to day 7 in the N1 and N2 groups, with the lowest WBC level observed on day 7. However, the WBCs gradually increased from day 7 to day 20 ( Figure 2A ). The decreasing and increasing levels of WBCs in the N1 and N2 groups were significantly different. On day 7, the percentage of WBCs in the blood of N2 mice was lower than in N1 mice (21.10% vs. 34.98%, respectively). The recovery rate of WBCs in N1 mice was more rapid than in N2 mice. In fact, after day 20, the level of WBCs in N1 mice reached 91.12%, which was similar to normal mice (106.08%). In contrast, the level of WBCs in N2 mice reached 51.33% only. Therefore, 20 mg/kg of BU in combination with 50 mg/kg of CY divided into 4 continuous injections over 4 days efficiently suppressed the murine immune system.
Although the level of WBCs remained rather stable in N2 mice, we next attempted to stabilize the level of WBCs with a maintenance dose of CY. Three treatment modes, N2a, N2b and N2c, were assessed. The results presented in Figure 2B show that the N2b mode optimally maintained a stable level of WBCs from day 7 to day 20.
Immune suppressed mouse models of hindlimb ischemia
To create a suitable mouse model of hindlimb ischemia, we first evaluated the effect of mouse age on the efficiency of immune suppression. Mice aged 3–5 months (group 1; 15 mice) and 6–12 months (group 2; 15 mice) were evaluated. The results showed significant differences in the degree of ischemic damage, pedal frequency, tissue edema, and histology of ischemic tissue between the two age groups.
In both groups, the blood flow could not go through the hindlimbs after burning the femoral arteries. After 1–2 h, the hind limbs of mice in both groups became livid and were not stained following injection with trypan blue, while hindlimb staining was observed in the normal mice ( Figure 3 ). Therefore, femoral artery burning blocked the blood flow to the hindlimbs in both groups of experimental mice.
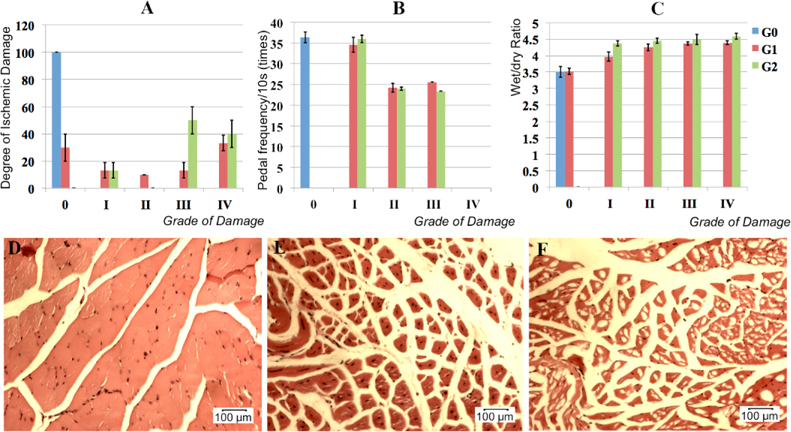
However, after 10 days, the degree of ischemic damage was different between the two groups. In the group 1 (3–5 months), 70% of the mice exhibited hindlimb ischemia that could be classified as: grade I, 20%; grade II, 10%; grade III, 10%; and grade IV, 30%. Thirty percent of the mice exhibited auto-recovery without hindlimb ischemia symptoms. In group 2 (6–12 months), 100% of the mice exhibited symptoms of hindlimb ischemia classified as: grade I, 10%; grade III, 50%; and grade IV, 40% ( Figure 4A ). According to these data, we used the 70% of mice with hindlimb ischemia in group 1 and all mice in the group 2 for further assays.
For the second assay, we evaluated the pedal frequency in water over a 10-s period for mice in group 1, group 2 and the control group. The natural response of mice placed in water is a pedal response. The pedal frequency for the control group was 40.67 ± 2.52 times/10s ( Figure 4B ). The pedal frequency in groups 1 and 2 was zero because of their hindlimb loss.
Three days after burning the arteries, the ratio of wet tissue to dry tissue was evaluated to record the tissue edema. These ratios in normal mice, group 1 mice and group 2 mice were 3.45 ± 0.09, 4.30 ± 0.09, and 4.59 ± 0.18, respectively ( Figure 4C ). Therefore, in the experimental groups (groups 1 and 2), tissue edema was significantly increased compared with normal mice. However, there was no statistically significant difference between the group 1 and 2 mice ( Figure 4C ).
Finally, histology was evaluated. Limb tissues were analyzed histologically after 4 weeks. After 72 h, muscles were dissected out, rinsed in PBS for 1 h, and then dehydrated. Samples were cut at cross sections 2 um in thickness and then stained with H-E. Compared with normal mice, significant histological differences were observed in muscles stained with H-E. In the group 1 and group 2 mice, the muscle cells became swollen after 72 h and then necrotic, as indicated by the destruction of nuclei, cytosol and cell membranes. In some mice at grade IV and V (in both groups 1 and 2), nuclei moved away from cell membrane into the cytosol, while in normal muscle cells the nuclei are found close to the membrane. In these mice, the muscle tissue also changed significantly, the associations between muscle cells were lost, and most cells were disconnected with huge spaces between them ( Figure 4D-F ). From these results, we chose mice 6–12 months in age for establishing the hindlimb ischemia models.
Efficiency of hindlimb ischemia treatment
Degree of ischemic damage in the different groups
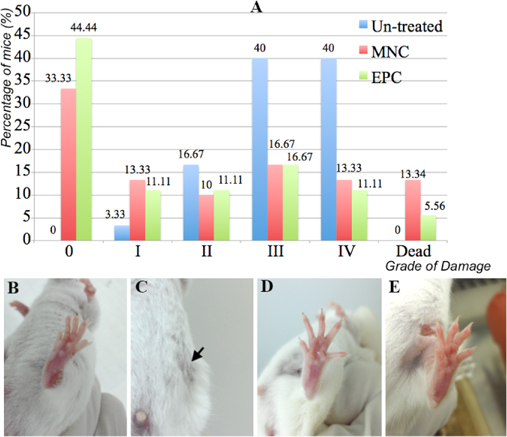
The damaged tissue was first evaluated to assess ischemic hindlimb healing. Figure 5 shows that MNC or EPC transplantation helped to improve the tissue damage toward normal (grade 0). In fact, in the untreated mice, 0% of grade 0 mice were observed after 20 days, while 33.33% (10/30) and 44.44% (8/18) of mice recovered toward grade 0. Moreover, the percentage of treated mice of grades II, III and IV also decreased (16.67% (5/30) vs. 10% (3/30) and 11.11% (2/18) at grade II; 40% (12/30) vs. 16.67% (5/30) and 16.67% (2/18) at grade III; 40% (12/30) vs. 13.33% (4/30) and 11.11% (2/18) at grade IV, respectively, for the untreated mice, MNC transplanted mice and EPC transplanted mice). Therefore, MNC or EPC transplantation significantly improved the tissue damage in ischemic hindlimbs. However, MNC and EPC transplantation also killed 13.34% (4/30) and 5.56% (1/18) of mice, respectively, compared with 0% for untreated mice.
We also recognized differences in the treatment efficiency of MNC and EPC transplantation. There was a significant increase in grade 0 mice following EPC transplantation compared with MNC transplantation (44.44% vs. 33.33%, respectively). EPC transplantation also decreased the percentage of dead mice from 13.34% in MNC transplanted mice to 5.56% in EPC transplanted mice. After 20 days of treatment, the percentages of mice at grade I, II, II and IV were the same (P < 0.05).
Pedal frequency and tissue edema
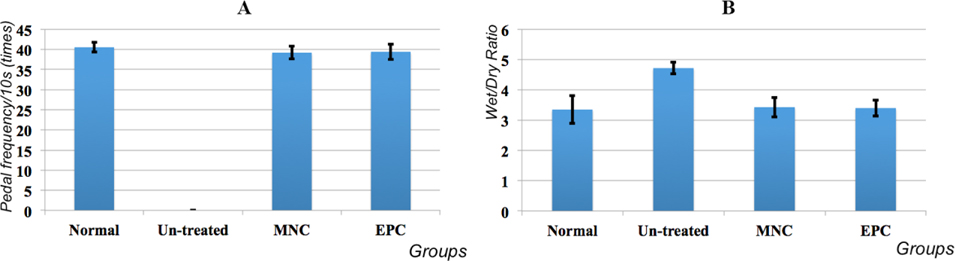
The functional recovery of the hindlimb is an important parameter for demonstrating full healing of ischemic hindlimbs. In this research, hindlimb function was evaluated by pedal frequency and tissue edema. The pedal frequency per 10s was compared between MNC-and EPC- transplanted mice whose tissue damage recovered to grade 0 with normal and un-treated mice. Ischemic hindlimb function was completely recovered in treated mice with tissue damage of grade 0. Regarding pedal frequency, all treated mice could stamp from 39.21 ± 1.54 times or 39.43 ± 1.89 times, respectively, following MNC- and EPC transplantation, compared with 40.55 ± 1.24 times in normal mice. These differences were not statistically significant ( Figure 6A ). Therefore, the pedal frequencies of MNC-and EPC- transplanted mice were similar to normal mice.
Tissue edema in treated mice of grade 0 strongly decreased to normal levels (3.35 ± 0.45), from 4.72 ± 0.19 in un-treated mice to 3.43 ± 0.32 in MNC-transplanted mice and 3.4 ± 0.26 in EPC-transplanted mice ( Figure 6B ).
Histology and neovascularization
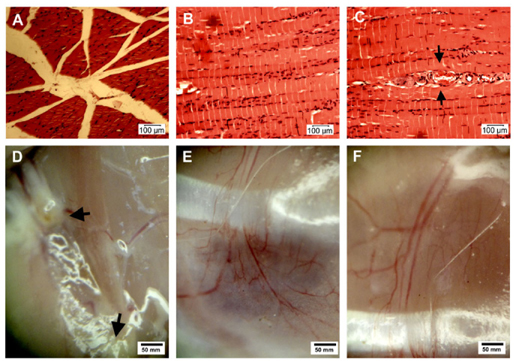
Treated mice of grade 0 were used for histological analysis, and the results are presented in Figure 7A-B . Muscle histology recovered fully, with healthy muscle bundles being observed. The muscle cells contained clear nuclei lying close to the cell membrane and homogeneous cytosol. The tissue bundles were linked together closely.
Moreover, 20 days after transplantation, many new blood vessels formed in both MNC-and EPC-transplanted mice. The new vessels connected the two sites of the femoral arteries that had been blocked by burning. Most of these blood vessels were newly formed by neovascularization (angiogenesis or vasculogenesis), because they were not detected in untreated mice ( Figure 7 ).
Discussion
Ischemia, particularly lower limb ischemia, has become common in recent years. Some therapies have been developed to treat this disease; however, their efficacy is limited. Stem cell therapy offers a new strategy for treating this disease. Unlike previous therapies, stem cell therapy leads to the regeneration or creation of new blood vessels to bypass the ischemic area. Therefore, such therapy is considered as the most efficacious for the treatment of ischemia.
In this research, we first isolated MNCs and EPCs from UCB. MNCs were isolated by gradient centrifugation, while EPCs were isolated from MNCs by sorting cells with CD133+ CD34+ marker expression. The composition of stem cells in MNCs was also analyzed by flow cytometry. We found that all MNC samples contained three kinds of stem cells, including MSCs, HSCs and EPCs. Sorted EPCs with CD133+ CD34+ marker expression were also expanded before transplantation. All samples of MNCs and EPCs were assessed for quality before transplantation based on the expression of stem cell markers.
Prior to transplantation of MNCs and EPCs, we established a model of hindlimb ischemia by immune suppression. Athymic nude mice or NOD/SCID mice were not used in this study because the aim was to establish an allogeneic model relevant to allogeneic grafts in humans. To induce acute hindlimb ischemia in mice, we suppressed the immune system before burning the femoral arteries. We successfully suppressed the immune system with BU and CY followed by a maintenance dose of CY. With this strategy, approximately 80% of white blood cells were reduced compared with before injection of BU and CY. Moreover, the low level of WBCs was maintained for 20 days, which was sufficient to carry out stem cell transplantation. Although BU and CY have long been used to establish immunesuppressed mouse models, we carefully evaluated and established a new protocol to efficiently suppress the immune system for stem cell transplantation and subsequent studies on ischemic hindlimb recovery. Next, we induced hindlimb ischemia in the immune-suppressed mice. Previously, we demonstrated two methods of blocking the blood flow in the femoral arteries, including burning the femoral arteries with electric equipment and femoral ligation. However, the success rate of femoral artery burning was higher than femoral ligation (data not shown). Hence, we used femoral artery burning in this research to establish hindlimb ischemia murine models. We successfully established hindlimb ischemia in 100% of mice when using mice 6–12 months in age.
MNC and EPC transplantation clearly improved ischemia in the model mice. Compared with untreated mice (injected with PBS), significant differences in all physiological characteristics and histology were observed. One of the indicators we were able to record easily to show recovery from ischemia was the degree of ischemic damage. In all MNC and EPC transplantation groups, the degree of ischemic damage was decreased. In almost all cases, ischemic tissue recovered, particularly after EPC transplantation, which enabled 44.44% of mice to escape tissue damage. This improvement was confirmed by the pedal frequency in water. After 20 days, all untreated mice lost limbs, whereas limbs were retained in all mice in the MNC and EPC transplantation groups. Leg movements in water demonstrated that muscle tissue was alive and functional. In other experiments, we recorded and compared tissue function in untreated and treated mice.
We found an improvement in tissue edema following transplantation. Mice treated with MNCs or EPCs showed significantly decreased tissue edema, and achieved the normal level of grade 0. This decrease showed that the blood vessels were smooth. Blood can enter ischemic tissue to enable cell recovery. Histological analysis supported these results. All muscle cells in untreated mice were killed by necrosis, whereas MNC and EPC transplantation improved cell survival. The pedal response in water confirmed this conclusion.
MNCs as well as EPCs may perform some roles in such regeneration. Analysis prior to injection showed that MNCs contained at least two kinds of stem cells, including MSCs and EPCs that play important roles in neovascularization and angiogenesis. Vasculogenesis is the process that forms vascular structures from circulating or tissue-resident endothelial stem cells (angioblasts) that proliferate into de novo endothelial cells. Angiogenesis describes the formation of thin-walled endothelial cell-lined structures with a smooth muscle wall and pericytes. These processes play vital roles during adult life as mechanisms to repair damaged tissues.
Many previous studies also confirmed that transplanted UCB-MSCs and EPCs exhibit angiogenic potential. Some studies demonstrated improvement of ischemic disease by transplanted UCB-MSCs Kim et al., 2006, Bhang et al., 2012, Zhang et al., 2012. Transplantation of hUCB-MSCs as spheroids significantly increased the number of microvessels and smooth muscle α-actin-positive vessels in the ischemic limbs of mice, and attenuated limb loss and necrosis Bhang et al., 2012. EPCs were also used to treat ischemia induced by diabetic injury with high benefits Shen et al., 2013. EPC gene therapy for hindlimb ischemia has also been studied. Yu et al. (2009) isolated EPCs from peripheral blood, transfected them with VEGF and transplanted them into ischemic hindlimbs with stromal derived factor 1 alpha (SDF-1 alpha) in a murine model. The results showed that the combination of SDF-1 alpha and VEGF greatly increased EPC-mediated angiogenesis Yu et al., 2009. UCB-derived EPCs cultured in medium supplemented with stem cell factor, FMS-like tyrosine kinase 3 ligand, interleukin-3 and basic fibroblast growth factor promoted the recovery of blood flow and neovascularization in ischemic hindlimb in vivo O et al., 2011. UCB-derived progenitor cells selected by culture on nano-fiber-based serum also exhibited neovascularization Das et al., 2009. UCB ALDH(hi) cells significantly enhanced the recovery from perfusion in ischemic limbs in femoral artery ligation mouse models Putman et al.,2012.
Both MSCs and EPCs contribute to new blood vessel formation, including neovascularization and angiogenesis, by two mechanisms. The first mechanism involves the differentiation of MSCs or EPCs into endothelial cells that participate in de novo vasculogenesis. UCB-MSCs can differentiate into functional endothelial cells and promote revascularization Xu et al., 2010. UCB EPCs transplanted via the tail vein into nude mice incorporated into capillary networks in ischemic hindlimbs, augmented neovascularization, and improved ischemic limb salvage. In addition, in ischemic tissue, expression of VEGF and SDF-1 alpha was observed, both of which displayed chemotactic effects on EPCs Yang et al., 2004.
The second mechanism involves de novo angiogenesis induced by factors secreted from MSCs or EPCs. Indeed, UCBderived progenitor cells selected from culture on nano-fiberbased serum also exhibited neovascularization. These cells expressed high levels of stem cell homing receptor, CXCR4, adhesion molecules and LFA-1. When grafted in a mouse hindlimb vascular injury model, they helped to restore blood flow Das et al., 2009. In another study, Shen et al. (2013) showed that EPCs improved diabetic hindlimb ischemia by overexpression of HIF-1 alpha/IL-8 Shen et al., 2013. In a recent study, Ratajczak et al. (2013) observed that CD133+ cells express mRNAs for several antiapoptotic and proangiopoietic factors, including kit ligand, insulin growth factor-1, vascular endothelial growth factor, basic fibroblast growth factor, and interleukin-8. These factors were also detected in CD133+ cell-derived conditioned medium (CM) Ratajczak et al., 2013. MSC CM and EPC CM promoted both endothelial cell adhesion and proliferation by simultaneously inducing the secretion of factors by MSCs or EPCs Burlacu et al., 2013. These factors also activated the survival protein, Akt, in cardiac myocytes and endothelial cells, which limits apoptosis and necrosis during hypoxia Henning et al., 2012.
In this study, we found that EPC transplantation was clearly more effective than MNC transplantation for ischemic hindlimb treatment. EPC transplantation significantly increased the percentage of fully recovered mice and decreased the percentage of dead mice. We propose two reasons for these results. First, although the MNCs contained some stem cell populations, they also contain many mature mononuclear cells. The existence of mature mononuclear cells can be harmful to the body, particularly the acute immune response to xenogenic cell antigens. Indeed, in both MNC-and EPC-transplanted mice, some mice died after transplantation while 100% of untreated mice remained alive. Importantly, following MNC transplantation, the percentage of dead mice was significantly higher than following EPC transplantation. Furthermore, the existence of mature mononuclear cells in MNCs would decrease the stem cell composition. Therefore, at the same dose of injected cells, the number of stem cells transplanted in EPCs will always be higher than in MNCs. Because of their pivotal role, a larger number of EPCs should demonstrate a better effect than the smaller stem cell population in the MNC fraction.
Second, there are two kinds of EPCs in UCB, which are referred to as circulating angiogenic cells (CACs) and high proliferative potential endothelial progenitor cells (HPPEPCs). Both types of cells express CD31, VE-cadherin, KDR and von Willebrand factor. CACs express CD14 but not CD133, while HPP-EPCs express CD133 but not CD14. HPPEPCs display stronger proliferation and clonogenic potential in vitro and show stronger ability to promote vascular growth in the hindlimb model of ischemia in mice in vivo than CACs Duan et al., 2006. In this study, we only used HPP-EPCs isolated by CD34+ CD133+ based sorting, which may have contributed to the transplantation efficiency and raised the percentage of fully-recovered mice.
Our results are also supported by some other studies. In 2010, Finney et al. compared the vasculogenic functionality between UCB EPCs (CD133+) and bone marrow-derived mononuclear cells. They showed that UCB CD133+ cells exhibit more robust vasculogenic functionality compared with BM-MNCs in response to ischemia Finney et al., 2010. However, cultured EPCs from UCB and BM exhibited equivalent neovascularization in vivo Finney et al., 2006. Previously, to investigate the roles of bone marrow-derived MSCs (BM-MSCs) and bone marrow-derived mononuclear cells (BM-MNCs), Iwase et al. (2005) showed that BM-MSC transplantation caused a significantly greater improvement in hindlimb ischemia than BM-MNC transplantation. Compared with BM-MNCs, BM-MSCs survived well in the ischemic environment and differentiated into not only endothelial cells but also vascular smooth muscle cells Iwase et al., 2005.
Conclusion
UCB is a rich source of stem cells. The MNC fraction or EPCs derived from this source of blood can improve hindlimb ischemia in murine models. Transplantation of both MNCs and EPCs clearly reduces ischemia in hindlimb ischemic mouse models, with a significant percentage of mice fully recovering with regard to both tissue damage and hindlimb function. MNCs and EPCs promote angiogenesis and vasculogenesis, in which many new blood vessels are formed to supply nutrients to muscle cells and other kinds of cells in the hindlimb. Such recovery involves not only the survival of muscle cells but also their physiological activities, particularly their motility. However, transplantation of purified EPCs was more effective than MNC transplantation, as shown by a higher percentage of fully recovered mice and a reduced percentage of dead mice following foreign cell transplantation. Taken together with their other advantages, UCB-EPCs are suitable candidates for treating ischemia by both autogenic and allogeneic transplantation. The results of this study promise new applications for banked UCB.
Abbreviations
HSCs: Hematopoietic stem cells; UCB: Umbilical cord blood; MSCs: Mesenchymal stem cells; EPCs: Endothelial progenitor cells; MNCs: Mononuclear cells; ALI: Acute hindlimb ischemia; BU: Busulfan; CY: cyclophosphamide; VEGF: Vascular endothelial growth factor; PBS: Phosphate buffered saline; 7-ADD: 7-amino actinomycin; CACs: Circulating angiogenic cells; TGF-Ά: transforming growth factor-beta; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor.
Authors’ contributions
All authors read and approved the final manuscript. NBV carried out studies including primary culture, isolation of MNCs, EPCs, stem cell transplantation. ANTB and LTP performed the murine models. ANTB, LTP and VNLT evaluated the stem cell transplantation efficiency. NKP participated in designing the study. PVP performed the EPC sorting, prepared the manuscript in cooperation with all other authors.
References
-
S.
Andaz,
D.A.
Shields,
J.H.
Scurr,
P.D.
Smith.
Thrombolysis in acute lower limb ischaemia. European journal of vascular surgery.
1993;
7
:
595-603
.
-
S.H.
Bhang,
S.
Lee,
J.Y.
Shin,
T.J.
Lee,
B.S.
Kim.
Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue engineering Part A.
2012;
18
:
2138-2147
.
-
A.
Burlacu,
G.
Grigorescu,
A.M.
Rosca,
M.B.
Preda,
M.
Simionescu.
Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem cells and development.
2013;
22
:
643-653
.
-
M.G.
Butler,
J.E.
Menitove.
Umbilical cord blood banking: an update. Journal of assisted reproduction and genetics.
2011;
28
:
669-676
.
-
K.
Callum,
A.
Bradbury.
ABC of arterial and venous disease: Acute limb ischaemia. BMJ (Clinical research ed).
2000;
320
:
764-767
.
-
S.W.
Cho,
S.J.
Gwak,
S.W.
Kang,
S.H.
Bhang,
K.W.
Won Song,
Y.S.
Yang,
C.Y.
Choi,
B.S.
Kim.
Enhancement of angiogenic efficacy of human cord blood cell transplantation. Tissue engineering.
2006;
12
:
1651-1661
.
-
L.
Chularojmontri,
S.K.
Wattanapitayakul.
Isolation and characterization of umbilical cord blood hematopoietic stem cells. Journal of the Medical Association of Thailand = Chotmaihet thangphaet 92 Suppl.
2009;
3
:
S88-94
.
-
L.
Corfield,
D.J.
McCormack,
R.
Bell,
P.
Taylor,
J.
Reidy.
Role of the femorofemoral crossover graft in acute lower limb ischemia due to acute type B aortic dissection. Vascular.
2013
.
-
H.
Das,
N.
Abdulhameed,
M.
Joseph,
R.
Sakthivel,
H.Q.
Mao,
V.J.
Pompili.
Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell transplantation.
2009;
18
:
305-318
.
-
B.
Delalat,
A.A.
Pourfathollah,
M.
Soleimani,
H.
Mozdarani,
S.R.
Ghaemi,
A.A.
Movassaghpour,
S.
Kaviani.
Isolation and ex vivo expansion of human umbilical cord blood-derived CD34+ stem cells and their cotransplantation with or without mesenchymal stem cells. Hematology.
2009;
(Amsterdam
:
Netherlands) 14, 125-132
.
-
H.X.
Duan,
L.M.
Cheng,
J.
Wang,
L.S.
Hu,
G.X.
Lu.
Angiogenic potential difference between two types of endothelial progenitor cells from human umbilical cord blood. Cell biology international.
2006;
30
:
1018-1027
.
-
H.X.
Duan,
G.X.
Lu,
L.M.
Cheng.
[Isolation, culture and identification of two types of endothelial progenitor cells from human umbilical cord blood]. Zhongguo shi yan xue ye xue za zhi / Zhongguo bing li sheng li xue hui = Journal of experimental hematology / Chinese Association of Pathophysiology.
2008;
16
:
387-391
.
-
M.R.
Finney,
L.R.
Fanning,
M.E.
Joseph,
J.L.
Goldberg,
N.J.
Greco,
S.
Bhakta,
D.G.
Winter,
M.
Forster,
P.E.
Scheid,
M.
Sabe.
Umbilical cord blood-selected CD133(+) cells exhibit vasculogenic functionality in vitro and in vivo. Cytotherapy.
2010;
12
:
67-78
.
-
M.R.
Finney,
N.J.
Greco,
S.E.
Haynesworth,
J.M.
Martin,
D.P.
Hedrick,
J.Z.
Swan,
D.G.
Winter,
S.
Kadereit,
M.E.
Joseph,
P.
Fu.
Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation.
2006;
12
:
585-593
.
-
I.J.
Fuss,
M.E.
Kanof,
P.D.
Smith,
H.
Zola.
Isolation of whole mononuclear cells from peripheral blood and cord blood. Current protocols in immunology / edited by John E Coligan [et al] Chapter.
2009;
7
:
Unit7 1
.
-
T.
Goto,
N.
Fukuyama,
A.
Aki,
K.
Kanabuchi,
K.
Kimura,
H.
Taira,
E.
Tanaka,
N.
Wakana,
H.
Mori,
H.
Inoue.
Search for appropriate experimental methods to create stable hind-limb ischemia in mouse. The Tokai journal of experimental and clinical medicine.
2006;
31
:
128-132
.
-
R.J.
Henning,
S.
Dennis,
D.
Sawmiller,
L.
Hunter,
P.
Sanberg,
L.
Miller.
Human umbilical cord blood mononuclear cells activate the survival protein Akt in cardiac myocytes and endothelial cells that limits apoptosis and necrosis during hypoxia. Translational research : the journal of laboratory and clinical medicine.
2012;
159
:
497-506
.
-
T.
Iwase,
N.
Nagaya,
T.
Fujii,
T.
Itoh,
S.
Murakami,
T.
Matsumoto,
K.
Kangawa,
S.
Kitamura.
Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovascular research.
2005;
66
:
543-551
.
-
T.
Jaatinen,
J.
Laine.
Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient. Current protocols in stem cell biology Chapter.
2007;
2
:
Unit 2A 1
.
-
Z.
Jaffery,
S.N.
Thornton,
C.J.
White.
Acute limb ischemia. The American journal of the medical sciences.
2011;
342
:
226-234
.
-
D.G.
Kent,
M.R.
Copley,
C.
Benz,
S.
Wohrer,
B.J.
Dykstra,
E.
Ma,
J.
Cheyne,
Y.
Zhao,
M.B.
Bowie,
Y.
Zhao.
Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood.
2009;
113
:
6342-6350
.
-
M.H.
Kim,
H.Z.
Zhang,
S.W.
Kim.
Combined growth factors enhanced angiogenic potential of cord blood-derived mononuclear cells transplanted to ischemic limbs. Journal of molecular and cellular cardiology.
2011;
51
:
702-712
.
-
S.W.
Kim,
H.
Han,
G.T.
Chae,
S.H.
Lee,
S.
Bo,
J.H.
Yoon,
Y.S.
Lee,
K.S.
Lee,
H.K.
Park,
K.S.
Kang.
Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem cells.
2006;
(Dayton
:
Ohio) 24, 1620-1626
.
-
D.R.
Li,
J.H.
Cai.
Methods of isolation, expansion, differentiating induction and preservation of human umbilical cord mesenchymal stem cells. Chinese medical journal.
2012;
125
:
4504-4510
.
-
R.Z.
Lin,
A.
Dreyzin,
K.
Aamodt,
A.C.
Dudley,
J.M.
Melero-Martin.
Functional endothelial progenitor cells from cryopreserved umbilical cord blood. Cell transplantation.
2011;
20
:
515-522
.
-
C.
Liu,
Z.
Sun,
X.
Du,
X.
Chen,
J.
Feng,
B.
Jia.
Implantation of endothelial progenitor cells into laser-induced channels in rat ischemia hindlimb augments neovascularization. Annals of vascular surgery.
2005;
19
:
241-247
.
-
S.P.
Lyden.
Endovascular treatment of acute limb ischemia: review of current plasminogen activators and mechanical thrombectomy devices. Perspectives in vascular surgery and endovascular therapy.
2010;
22
:
219-222
.
-
L.E.
Mead,
D.
Prater,
M.C.
Yoder,
D.A.
Ingram.
Isolation and characterization of endothelial progenitor cells from human blood. Current protocols in stem cell biology Chapter.
2008;
2
:
Unit 2C 1
.
-
T.
Murohara,
H.
Ikeda,
J.
Duan,
S.
Shintani,
K.
Sasaki,
H.
Eguchi,
I.
Onitsuka,
K.
Matsui,
T.
Imaizumi.
Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. The Journal of clinical investigation.
2000;
105
:
1527-1536
.
-
F.
Notta,
S.
Doulatov,
E.
Laurenti,
A.
Poeppl,
I.
Jurisica,
J.E.
Dick.
Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science (New.
2011;
York
:
NY) 333, 218-221
.
-
E.
O,
B.H.
Lee,
H.Y.
Ahn,
J.C.
Shin,
H.K.
Kim,
M.
Kim,
I.Y.
Park,
Y.G.
Park,
Y.A.
Joe.
Efficient nonadhesive ex vivo expansion of early endothelial progenitor cells derived from CD34+ human cord blood fraction for effective therapeutic vascularization. FASEB journal : official publication of the Federation of American Societies for Experimental Biology.
2011;
25
:
159-169
.
-
P.V.
Phuc,
V.B.
Ngoc,
D.H.
Lam,
N.T.
Tam,
P.Q.
Viet,
P.K.
Ngoc.
Isolation of three important types of stem cells from the same samples of banked umbilical cord blood. Cell and tissue banking.
2012;
13
:
341-351
.
-
P.M.
Pimentel-Coelho,
P.H.
Rosado-de-Castro,
L.M.
da Fonseca,
R.
Mendez-Otero.
Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatric research.
2012;
71
:
464-473
.
-
D.M.
Putman,
K.Y.
Liu,
H.C.
Broughton,
G.I.
Bell,
D.A.
Hess.
Umbilical cord blood-derived aldehyde dehydrogenaseexpressing progenitor cells promote recovery from acute ischemic injury. Stem cells.
2012;
(Dayton
:
Ohio) 30, 2248-2260
.
-
J.
Ratajczak,
M.
Kucia,
K.
Mierzejewska,
W.
Marlicz,
Z.
Pietrzkowski,
W.
Wojakowski,
N.J.
Greco,
M.
Tendera,
M.Z.
Ratajczak.
Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells-implications for stem cell therapies in regenerative medicine. Stem cells and development.
2013;
22
:
422-430
.
-
W.C.
Shen,
C.J.
Liang,
V.C.
Wu,
S.H.
Wang,
G.H.
Young,
I.R.
Lai,
C.L.
Chien,
S.M.
Wang,
K.D.
Wu,
Y.L.
Chen.
Endothelial Progenitor Cells Derived from Wharton’s Jelly of the Umbilical Cord Reduces Ischemia-Induced Hind Limb Injury in Diabetic Mice by Inducing HIF-1alpha/IL-8 Expression. Stem cells and development.
2013
.
-
E.
Song,
C.W.
Lu,
L.J.
Fang,
W.
Yang.
Culture and identification of endothelial progenitor cells from human umbilical cord blood. International journal of ophthalmology.
2010;
3
:
49-53
.
-
A.F.
Sousa,
P.Z.
Andrade,
R.M.
Pirzgalska,
T.M.
Galhoz,
A.M.
Azevedo,
C.L.
da Silva,
M.R.
Aires-Barros,
J.M.
Cabral.
A novel method for human hematopoietic stem/progenitor cell isolation from umbilical cord blood based on immunoaffinity aqueous two-phase partitioning. Biotechnology letters.
2011;
33
:
2373-2377
.
-
T.G.
Walker.
Acute limb ischemia. Techniques in vascular and interventional radiology.
2009;
12
:
117-129
.
-
J.M.
Wardlaw,
C.P.
Warlow.
Thrombolysis in acute ischemic stroke: does it work?. Stroke; a journal of cerebral circulation.
1992;
23
:
1826-1839
.
-
Y.
Xu,
H.
Meng,
C.
Li,
M.
Hao,
Y.
Wang,
Z.
Yu,
Q.
Li,
J.
Han,
Q.
Zhai,
L.
Qiu.
Umbilical cord-derived mesenchymal stem cells isolated by a novel explantation technique can differentiate into functional endothelial cells and promote revascularization. Stem cells and development.
2010;
19
:
1511-1522
.
-
C.
Yang,
Z.H.
Zhang,
Z.J.
Li,
R.C.
Yang,
G.Q.
Qian,
Z.C.
Han.
Enhancement of neovascularization with cord blood CD133+ cell-derived endothelial progenitor cell transplantation. Thrombosis and haemostasis.
2004;
91
:
1202-1212
.
-
J.X.
Yu,
X.F.
Huang,
W.M.
Lv,
C.S.
Ye,
X.Z.
Peng,
H.
Zhang,
L.B.
Xiao,
S.M.
Wang.
Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. Journal of vascular surgery.
2009;
50
:
608-616
.
-
H.C.
Zhang,
X.B.
Liu,
S.
Huang,
X.Y.
Bi,
H.X.
Wang,
L.X.
Xie,
Y.Q.
Wang,
X.F.
Cao,
J.
Lv,
F.J.
Xiao.
Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem cells and development.
2012;
21
:
3289-3297
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 01 (2014)
Page No.: 9-20
Published on: 2014-02-07
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8789 times
- Download PDF downloaded - 1764 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress