Evaluating the ameliorative potential of plant flavonoids and their nanocomposites in bleomycin induced idiopathic pulmonary fibrosis
Abstract
Introduction: Pulmonary Fibrosis can severely disrupt lung function. The cause of Idiopathic Pulmonary Fibrosis (IPF) is still unclear. The treatment currently available for IPF provides minimal benefits. Bleomycin is used in research to induce experimental pulmonary fibrosis in animals. Plant flavonoids such as fisetin and curcumin are known for their anti-inflammatory properties. The aim was to demonstrate the path of physiological changes in bleomycin induced IPF and to further evaluate the reversal and ameliorative potentials of some plant flavonoids and their nanocomposites. Bleomycin administration is known to damage the lung epithelial cells resulting in lower cell numbers.
Results: Our colony forming assay in BALF also demonstrated a reduction (1.65 fold) in colony-forming unit after bleomycin administration. The number of colonies increased upon treatment with fisetin(1.20 fold) and curcumin (1.13 fold) respectively. MTT proliferation assay demonstrated a significant increase in the number of viable cells upon treatment with curcumin (1.42 fold) and fisetin (1.74 fold) respectively, in the lung. The NBT assay demonstrated a significant effect of curcumin in BALF, lung and peripheral blood. A significant effect of fisetin was observed in BALF and peripheral blood.
Conclusion: These findings were also supported by the histological analyses of the lung samples. Curcumin showed highestpotential for the treatment of IPF and fisetin showed its ameliorative effect.
Introduction
Pulmonary fibrosis is the end result of a heterogeneous group of disorders, of known or unknown etiology, characterized by the destruction of functional lung parenchyma and its conversion to scar tissue Wilson and Wynn, 2009. This results in the replacement of lung tissue by dense fibrous connective tissue. This damage causes patients’ lungs to thicken and makes respiration difficult upon physical exertion. This can lead to severe decline in lung function, dyspnoea and death Antoniou et al., 2009Antoniou and Wells, 2008Maher et al., 2007. In recent years, its prevalence and annual incidence rate has increased significantly. It is the most common interstitial lung disease, which affects over five million people worldwide with a mean survival time of approximately three years. Pulmonary fibrosis is associated with some pitfalls, such as bacterial infection, inhalation of organic and inorganic dusts, environmental exposure, radiation, smoking, genetic factors, diabetes mellitus, infectious agents and trauma Antoniou et al., 2009Antoniou and Wells, 2008Maher et al., 2007Shi et al., 2014Wilson and Wynn, 2009. However, in cases of unknown etiology, it is referred to as Idiopathic Pulmonary Fibrosis (IPF). It is suggested that the cycle of chronic inflammations leading to fibrosis, initiated by these unrevealed insults, play a major role in the pathogenesis of IPF Akgedik et al., 2012. It may be that in response to injury, after entering the lung, inflammatory cells together with resident lung cells, release mediators for the stimulation of fibroblast proliferation and deposition of collagen within the lung interstitium Wilson and Wynn, 2009.
Repair of the damaged tissue is a fundamental biological mechanism. Dysregulation of this process can lead to the development of a permanent fibrotic scar. The pathological findings in pulmonary fibrosis are a consequence of disturbances in two physiologically balanced processes, such as, proliferation and apoptosis of fibroblasts, along with accumulation and breakdown of extracellular matrix (ECM) Todd et al., 2012. The precise mechanism for the development of fibrosis remains poorly understood Todd et al., 2012. Oxygen radicals leading to epithelial injury is one of the possible mechanisms in the pathogenesis of IPF Walters et al., 2008. Despite the severity of the disease, the current treatment for pulmonary fibrosis provides minimal benefits and has significant side effects, highlighting the need for novel treatment approaches.
Bleomycin (bleo) is used for induction of experimental pulmonary fibrosis in animals. Although the exact mechanisms by which bleomycin causes pulmonary fibrosis remains unclear, it has been reported that, Reactive Oxygen Species (ROS) generated by bleomycin, causes direct injury to the lung epithelial cells Banerjee et al., 2015Hay et al., 1991Wang et al., 2002. Reactive Oxygen Species such as superoxide anions, hydrogen peroxides, and hydroxyl radicals have been reported to play an important role as a mediator of bleo-induced lung fibrosis Arslan et al., 2002Chen and Stubbe,2004.
Due to the various side effects of modern medicines, the use of natural products with antioxidant properties is gaining popularity. Different phytochemicals originating from fruits are being exploited as possible sources of therapeutic agents. Phytochemicals act against diseases by up-regulating gene expressions of the antioxidant enzymes Aruoma, 1994Kumaravelu et al., 1995Verma et al., 2013. Fisetin (3, 7, 3′, 4′- tetrahydroxyflavone) belongs to the flavonoid group which are found in many plants, fruits and vegetables. The polyphenol, curcumin, Anand et al., 2007 is derived from the rhizome of the plant Curcuma longa. Curcumin has a long history of use in traditional medicines of some countries such as India and China Anand et al., 2007. Curcumin is known for its wide range of anti-inflammatory and antioxidant properties. Additionally, nanomaterials used as nanodrugs or as nano-vehicles, have the advantage of being small devices which can be targeted to reach a particular site. Nanomaterials have shorter biochemical reaction times, faster delivery and can be implanted inside the body Boisseau and Loubaton, 2011Kaur et al., 2011Mohamud et al., 2013. Therefore, we also used some nanocomposites in this present study.
Bleomycin is used for induction of experimental pulmonary fibrosis in animals Moeller et al., 2008, which is a popular model for the study of human lung fibrosis. In the light of promising properties and broad spectrum of activities of fisetin and curcumin, the aim of this study was to demonstrate the path of physiological changes in bleomycin-induced IPF and to further evaluate the reversal and ameliorative potential of these plant flavonoids and their nanocomposites. Bleomycin-induced changes in mice with idiopathic pulmonary fibrosis were used as a model for this recent study.
Methods
Animals
Balb/C mice were obtained from National Institute of Nutrition (NIN) Hyderabad, India. All mice were housed in the animal house of the Department of Zoology, University of Calcutta, India under pathogen-free conditions and were routinely given food and water. All experiments were performed according to rules laid down by the Institutional and departmental animal ethics committee of the University of Calcutta, Kolkata.
20 mice were randomly divided into five experimental groups with four animals each. Mice were anaesthetized using Propofol, The control group animals received an intratracheal injection of normal saline alone. The bleomycin group animals were subjected to a single intratracheal and intranasal instillation of bleomycin (0.075 U/ml). The bleomycin plus fisetin, bleomycin plus curcumin and bleomycin plus MCN plus fisetin group of animals were considered as a preventive model, which received the same amount of bleomycin as the bleomycin group animals and were then treated with fisetin 2μM/kg body weight (40 μl), curcumin 2μM/kg body weight (40 μl) and MCN treated fisetin 5.1mg/ml (40 μl) intratracheally at day 7, 14 and 21, respectively. Their body weights were recorded according to RHMP.
Twenty-eight days after the bleomycin treatment, the animals were sacrificed. Lung, bone marrow, spleen, bronchoalveolar lavage, and peripheral blood were collected.
The lung tissues were divided into pieces, one part was immersed in 10% formalin solution for histopathological examination, and one part was immersed in media for conducting inflammatory assay, cell proliferation assay and for total cell count, inner content of bone marrow was flushed out and the inner content of spleen was squeezed out for estimation of total cell count. Peripheral blood was collected for total cell count, cell proliferation and inflammatory assay.
Bronchoalveolar lavage fluid collection and analysis
The mouse lungs were lavaged three times with 1 ml of PBS. The retained BALF was centrifuged at 400 x g for 5 min at 4°C. The supernatant was collected and stored at -70°C for further analysis. The cell pellets were resuspended in culture media and used for different assays.
Colony Forming Unit (CFU)-c assay
For quantification of committed progenitors of all lineages, Colony-Forming Units in culture (CFU-c) was performed. Briefly, after dissection, BALF was extracted and immediately stored in Iscove’s Modified Dulbecco’s Media (IMDM) (Himedia, India), maintaining aseptic conditions. CFU-c media was prepared using IMDM, supplemented with 30% FBS (Himedia, India), 10% BSA (Biosera, India), 1% Penicillin-Streptomycin (Himedia, India) and 5 ng/ml murine SCF (Biovision, India). Lastly, 1.5% methylcellulose (Himedia, India) was added into the concoction. 1 ml CFU-c assay media and 500 μl cell suspension containing 1x105 cells were seeded in each well in a 24-well tissue culture plate. The cells were incubated at 37°C for 14 days. All Colony types were counted using Floid Cell Imaging Station (Life Technologies, India) and the numbers were pooled to get the total CFU-c.
Total Cell Count
A total cell count gives the information about the cell count of each cell types and the concentration of various proteins and minerals. The cells that circulate in the bloodstream are generally divided into three types: white blood cells (leukocytes), red blood cells (erythrocytes), and platelets thrombocytes. Abnormally high or low counts may indicate the presence of many forms of disease. Total cell count of peripheral blood, bone marrow, spleen, BALF and lung sample was estimated. The cell suspension was mixed in equal volume with trypan blue dye (Himedia, India) and counted using a hemocytometer.
Cell Proliferation Assay (MTS Assay)
The MTS assay is a colorimetric method for determining the number of viable cells in culture. The assay was standardized using various numbers of cells (0.5 x 105, 1.0 x 105, 0.5 x 106, 1.0 x 106, 0.5 x 107 and 1.0 x 107 cells per well), using the Promega CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay Kit (Promega, India). 100 μl of cells (PB, lung and BALF) from all the samples were seeded in a 96-well plate, and incubated for 1 hour in a CO2 incubator at 37°C. Following this, 20 μl of MTS/PMS solution was added to each well, and incubated in a CO2 incubator for 1-4 hours. Absorbance was measured immediately at 490 nm using a plate reader (Thermo-Fisher Scientific). The number of cells obtained using the standard curve was plotted against each sample.
Determination of Nitric Oxide Content (NO Assay)
Activation of immune system is associated with increase in macrophage NO production. Transient nature of NO makes it unsuitable for detection, but it is oxidized to Nitrite (NO2-) and Nitrate (NO3-) by nitrate reductase. Nitrite, a sub product of NO metabolism, was measured using the Griess reaction. Samples were treated with 50 mL of 1% sulfanilamide solution(SRL, India)for 10 minutes and mixed with 50 mL of 0.1% naphthyl ethylenediamine solution (SRL, India). Formation of the stable azo compound of purple color was measured spectrophotometrically by measuring the absorbance at 540 nm. The method was standardized with known concentrations of nitrite.
Determination of superoxide generation
A semi-quantitative microscopic nitro blue tetrazolium (NBT) assay was used to determine the production of superoxide anion [O2(-)] in various phagocytic cells. This microscopic assay is based on the formation of blue NBT formazan deposits from the reduction of the membrane permeable, water-soluble, yellow-colored, nitro blue tetrazolium (Y-NBT) by O2(-). Samples were treated with NBT solution (Himedia, India) for an hour, then the blue formazan particles were dissolved using 2M potassium hydroxide (Merck, India) and dimethylsulfoxide (Himedia, India). The absorbance was then measured using a micro plate reader (Thermo-Fisher Scientific) at 620 nm.
Estimation of cyclic AMP concentration
Adenosine 3’,5’-cyclic monophosphate (cyclic- AMP/cAMP) is an important ‘second messenger’ involved in many physiological processes. The concentration of cyclic AMP was measured by BioVision’s cAMP Assay Kit. Lung tissue sample was homogenized and then spun at highest speed for 5 min and the supernatant was collected. The assay was performed according to manufacturer’s protocol. The absorbance was measured at 450 nm.
Histology
After sacrificing the mice using cervical dislocation, one part of the lungs was carefully excised and fixed in 10% (w/v) PBS-buffered (Himedia, India) formaldehyde solution for one week at room temperature. The tissue was dehydrated using graded ethanol and paraffin embedded. 5 μm thick tissue sections were cut using a microtome (RM-2135, Leica Microsystems, Bensheim, Germany). To evaluate the histopathological changes, the sections were stained with haematoxylin and eosin (Himedia, India) staining. To identify the density of the accumulated collagen fibers, the sections were stained with Masson’s trichrome stain (Himedia, India).
Statistical analysis
All the differences were assessed by 1-tail Student’s ttest, one-way at α=5% using Graph Pad Prism 6. The results have been given as means ± SD.
Results
In this study, we have used a bleomycin-induced lung injury model to demonstrate if fisetin, curcumin, and MCN treated fisetin could be used as potential drugs for IPF.
Clonogenic potential assay of bronchoalveolar lavage fluid (BALF) sample of different groups
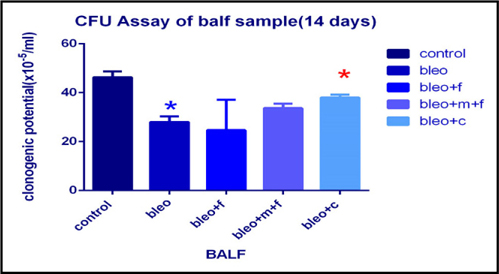
Clonogenic potential of BALF was estimated using colony forming assay as shown in Figure 1 . We found that, clonogenic potential of bleomycin treated sample decreased 1.65 fold as compared to control which was statistically significant. The count decreased 1.87 fold with fisetin, 1.37 fold with MCN treated fisetin, and 1.21 fold with curcumin respectively, as compared to the control sample. We observed a 1.20 fold increase upon treatment with curcumin, 1.35 fold increase with MCN treated fisetin and 1.13 fold with fisetin as compared to bleomycin treated samples. Moreover, the increase upon treatment with curcumin as compared to bleomycin treated sample was statistically significant. Overall, we found that bleomycin effectively inhibited the cellular recruitment and treatment with fisetin, and curcumin increased the cellular recruitment in BALF.
Microscopic observation of colony formation
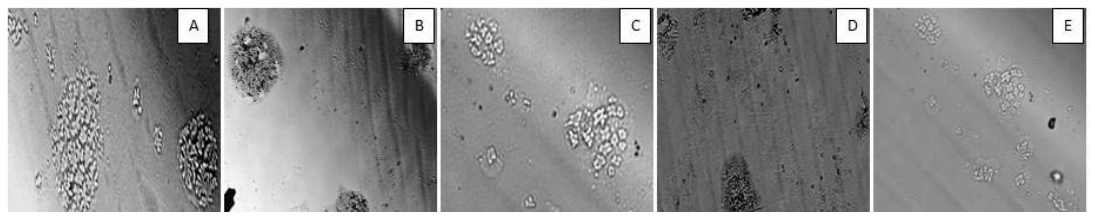
The colonies formed in the colony forming assay were also examined microscopically as illustrated in Figure 2 . This again demonstrated that, colony formation was reduced after bleomycin treatment, as the cells lost their clonogenic potential for colony formation. After treatment with the plant flavonoids and their nanocomposites, cells regained their clonogenic potential.
Total cell count of lung sample of treatment groups
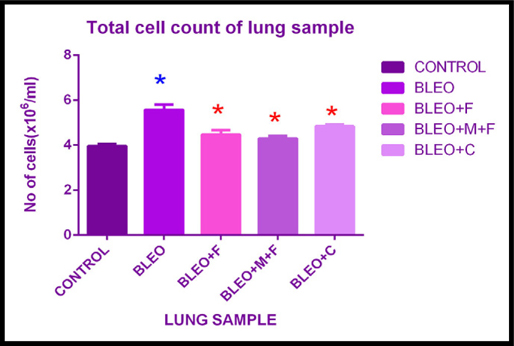
The total cell count was estimated for all the treatment groups using a hemocytometer. We found that, there was a 1.4 fold increase in the total cell count in bleomycin treated groups as compared to the control group. The count increased 1.12 fold with fisetin, 1.08 fold with MCN treated fisetin, and 1.10 fold with curcumin treatment as compared to the control. We observed a 1.24 fold decrease with MCN treated fisetin, 1.27 fold decrease with curcumin and 1.24 fold decrease with fisetin treatment as compared to bleomycin treated sample. This decrease in total cell count on treatment with flavonoids was statistically significant. Here we observed that, bleomycin was highly effective in causing fibrosis in mice as compared to control groups and the plant flavonoids showed their anti-fibrotic and anti-inflammatory action.
Total cell count of bronchoalveolar lavage fluid of different groups
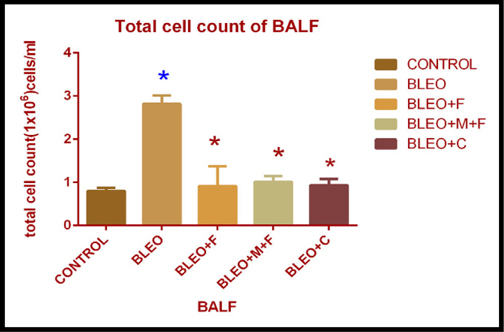
On examination of the total cell count in BALF, we found that there was a 3.5 fold increase in the total cell count in the bleomycin treated groups as compared to the control. The count increased 1.12 fold with fisetin, 1.2 fold with MCN treated fisetin, and 1.16 fold with curcumin treatment as compared to the control. A 2.7 fold decrease was observed with MCN treated fisetin, 3.02 fold decrease with curcumin and 3.09 fold decrease with fisetin treatment as compared to bleomycin treated samples. This decrease in total cell count on treatment with flavonoids was statistically significant. We found that, the inflammatory cell count was greatly increased for bleo treated individuals and effectiveness of fisetin was increased after addition of MCN with it, curcumin also showed its anti-inflammatory effects. Figure 3A
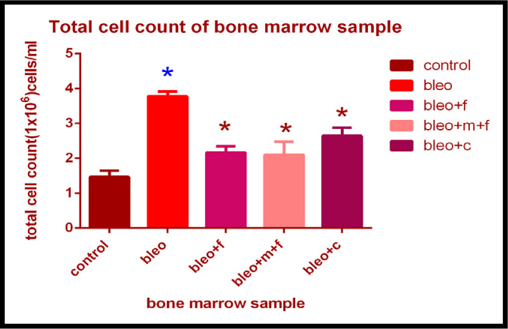
Total cell count of bone marrow sample of different groups
Similar to lung and BALF, total cell count in bone marrow sample showed a 2.5 fold increase in bleomycin treated groups as compared to the control. The cell count increased 1.47 fold with fisetin, 1.43 fold with MCN treated fisetin, and 1.80 fold with curcumin treatment as compared to the control. We found a 1.8 fold decrease in the cell count on treatment with MCN treated fisetin; a 1.42 fold decrease with curcumin and 1.74 fold decrease with fisetin treatment as compared to bleomycin treated sample. Again the decrease on treatment with fisetin and curcumin was statistically significant. Figure 3B
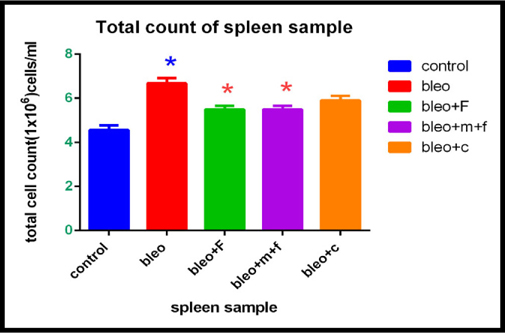
Total cell count of spleen sample of different groups
On estimating the total cell count for spleen we found that, there was a 1.46 fold increase in the total cell count in bleomycin treated groups as compared to the control. The count increased 0.04 fold with fisetin and MCN treated fisetin, and 1.29 fold with curcumin treatment as compared to the control. A 1.21 fold decrease was observed in both MCN treated fisetin and fisetin treated group as compared to bleomycin treated group, which was also statistically significant. We found a 1.13 fold decrease in the curcumin treated group as compared to only bleomycin treated group. However, this difference was not statistically significant. Figure 3C Figure 3D
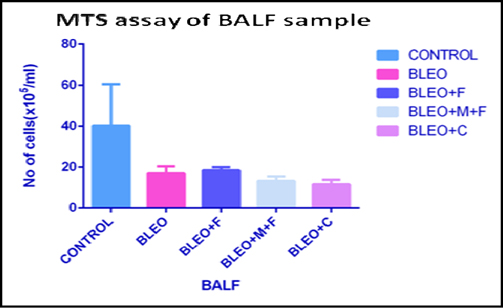
MTS cell proliferation assay of BALF sample of different groups
The MTS assay is an indicator of the proliferative potential of the cells. We found that, the cell number in the bronchoalveolar lavage fluid decreased 2.5 fold with bleomycin administration as compared to untreated control group. We observed that, there was a 1.47 fold decrease with Fisetin treatment, 1.43 fold decreases with MCN treated fisetin treatment, and a 1.80 fold decrease in cell number with curcumin treatment as compared to the untreated group. However, as compared to only bleomycin treated samples, we found a 1.8 fold increase with MCN treated fisetin, 1.42 fold increase with curcumin and 1.74 fold increase in cell numbers with fisetin treatment. Figure 4A
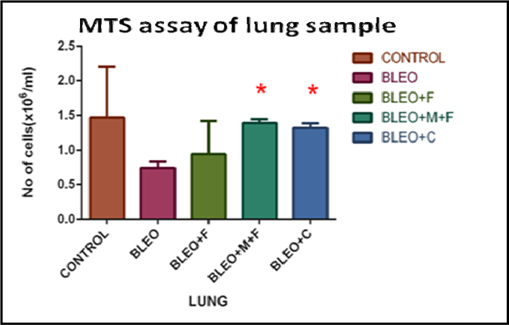
MTS cell proliferation assay of lung sample of different groups
By performing MTS assay on the hmg sample we observed that, the cell numbers decreased 2 fold with bleomycin treatment, as compared to untreated control group. On comparison of flavonoid treated samples with the control group, we found a 1.56 fold decrease with fisetin treatment, a 1.05 fold decrease with MCN treated fisetin treatment, and a 1.13 fold decrease in cell number with curcumin treatment. Further, a 1.8 fold increase in cell numbers with MCN treated fisetin treatment, 1.75 fold increase with curcumin treatment and 2 fold increase in cell number with fisetin treatment was observed as compared to only bleomycin treated samples. Figure 4B
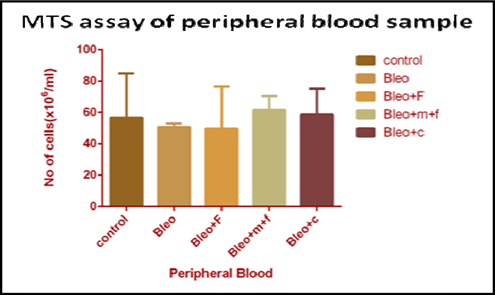
MTS cell proliferation assay of peripheral blood sample of different groups
In peripheral blood, the cell number decreased on bleomycin treatment, as compared to untreated control group. We found a 1.11 fold decrease with bleomycin treatment and a 1.14 fold decrease in cell number with fisetin treatment, as compared to the untreated group. However, there was a 1.08 fold and 1.03 fold increase in cell number with MCN treated fisetin and curcumin treatment as compared to the control group, respectively. A 1.12 fold increase with MCN treated fisetin and a 1.2 fold increase in cell number with curcumin treatment as compared to only bleomycin treated samples, but the cell count decreased on fisetin (1.02 fold) as compared to only bleomycin treatment. Figure 4C
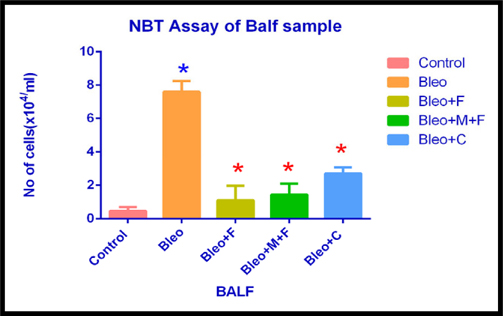
NBT Assay of BALF sample of different groups
Next, we performed NBT assay on samples to assess the inflammatory cell count in the treated and untreated samples. In the bronchoalveolar lavage fluid, the inflammatory cell count increased significantly (16.42 fold) after bleomycin administration. We observed a 2.39 fold, 3 fold and 5.8 fold increase in cell count with fisetin, MCN treated fisetin and curcumin treated samples, respectively, as compared to the control group. Inflammatory cell count decreased 7 fold with fisetin, 5.31 with MCN treated fisetin and 3 fold with curcumin, respectively as compared to only bleomycin treated samples. Here we found that, plant flavonoids used in this study, along with their nanocomposites, significantly reduced the inflammatory cell count.
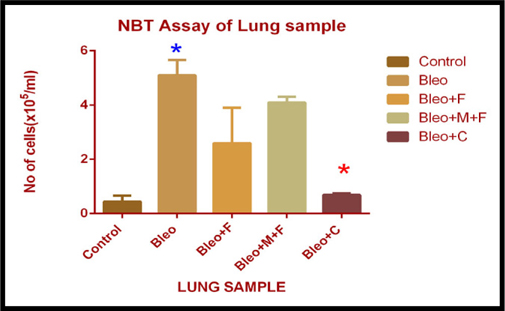
NBT Assay of lung sample of different groups
In lung sample, we observed a significant increase in the cell count (12 fold) in bleomycin a 9 fold with MCN treated fisetin, a 6 fold with fisetin and a 1.5 fold with curcumin treated group as compared to the untreated control group. Inflammatory cell count significantly decreased by 7.39 fold after administering curcumin, 2 fold with fisetin and 1.24 fold with MCN loaded fisetin treatment as compared to only bleomycin treated samples. Significant reduction in cell number was observed only after treatment with curcumin. Figure 5A
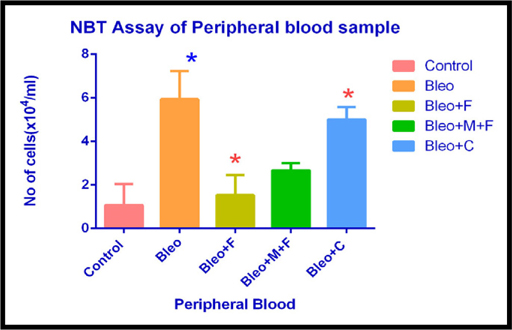
NBT Assay of peripheral blood sample of different groups
In peripheral blood sample, inflammatory cell count increased by 5 fold with bleomycin, 3.8 fold with fisetin, 5 fold with curcumin and 2.5 fold with MCN treated fisetin treatment as compared to the untreated group. Inflammatory cell count was significantly reduced after the treatment with flavonoid compounds. Curcumin was the most effective flavonoid in this assay.
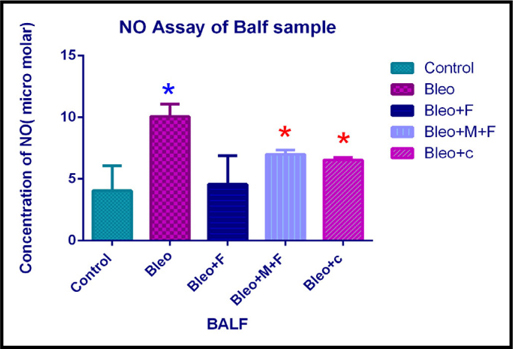
Estimation of nitric oxide concentration of BALF sample of different groups.
Nitric oxide (NO) is produced by macrophages as a defense mechanism against oxidative stress. In case of inflammation, the NO concentration is expected to rise, and again decrease with any anti- inflammatory agent.
NO content in our samples demonstrated that, the concentration of nitric oxide in bronchoalveolar fluid increased 2.5 fold with bleomycin, 1.42 fold with MCN treated fisetin, 1.53 fold with curcumin and 1.12 fold with fisetin treatment as compared to the untreated control group. However, on comparing with bleomycin treated samples, we found that, nitric oxide concentration decreased significantly by 2.2 fold with fisetin, 1.43 fold with MCN treated fisetin and 1.53 fold with curcumin treatment. Figure 5B
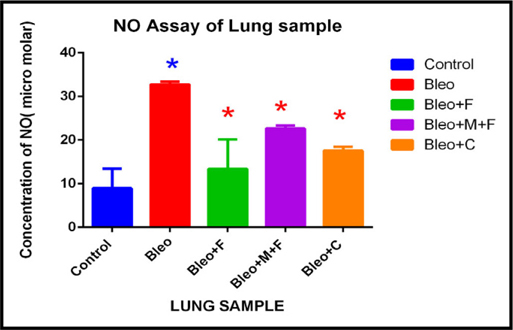
Estimation of nitric oxide concentration of lung sample of different groups.
Upon analysis of lung samples, we found that, the nitric oxide concentration increased by 3.65 fold after administration of bleomycin, 1.5 fold with fisetin, 2 fold with curcumin and 2.5 fold with MCN treated fisetin as compared to the control group. Nitric oxide concentration decread by 1.87 fold with curcumin and 1.45 fold with MCN treated fisetin as compared to only bleo. This finding suggests that, two plant flavonoids decreased the nitric oxide concentration at cellular level and their activity was greatly enhanced by the addition of nanocomposites. Figure 5C Figure 6A
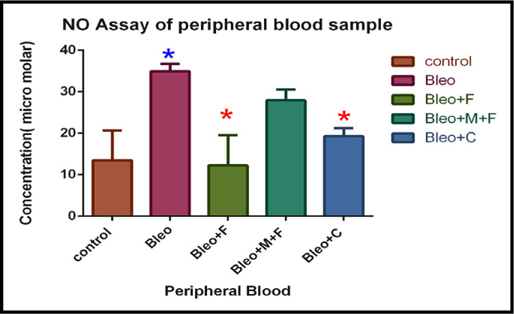
Estimation of nitric oxide concentration of peripheral blood sample of different groups.
In peripheral blood sample, nitric oxide concentration significantly increased (2.6 fold) on bleomycin treated sample, 2 fold with MCN treated fisetin, 1.43 with curcumin treatment as compared to the untreated control group. For fisetin treated group, NO concentration was decreased by 2.8 fold, and after curcumin treatment it was decreased by 1.8 fold. Figure 6B
Estimation of cyclic –AMP
TheCyclic-AMP concentration was determined in the different groups. We found that, the cAMP concentration was significantly increased by 1.84 fold after administration of bleomycin, and decreased by 1.2 fold with fisetin, 3.13 fold with MCN treated fisetin, and same with curcumin treatment as compared to the control. Figure 6C Figure 7
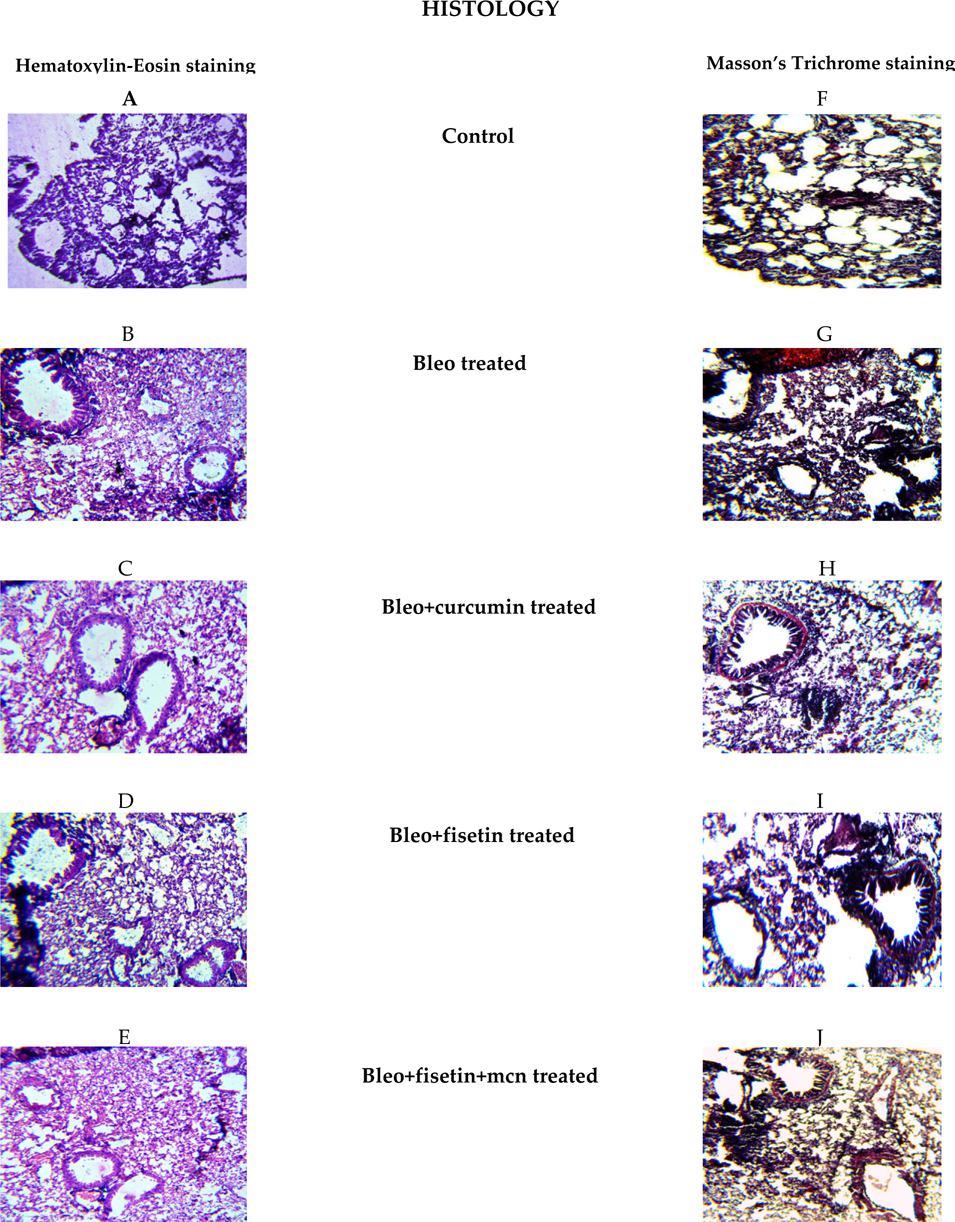
Histological assessment of lung sample of treatment groups
Hematoxylin and Eosin staining was performed to examine the histology of the treatment groups. We found that, the lung tissue section of bleomycintreated animals showed histopathological abnormalities as shown in Figure 8 . These include disturbance in alveolar structure, extensive thickening of the intra-alveolar septa, and dense interstitial inflammatory cells and fibroblast. None of these changes were observed in the control group. In contrast, the flavonoid treatment provided protection against bleomycin-induced lung tissue distortion. Significant amelioration was observed in the cellular infiltrates and thin lined alveolar septa was in the lung morphologies of the fisetin, curcumin and MCN treated fisetin treated groups compared to the bleomycin treated animals.
Masson’s-trichrome staining showed that the bleomycin treated animals had abnormal collagen deposition and distorted lung morphologies as compared to the control groups. Treatment with these compounds inhibited the extent and intensity of collagen, compared to the bleomycin treated samples. No such abnormalities were apparent in the tissues obtained from control animals.
Discussion
Here, we examined the effect of flavonoids namely, fisetin and curcumin in a mouse model of Idiopathic Pulmonary Fibrosis. Additionally, the effect of mesoporous carbon nanoparticles loaded with fisetin was analyzed.
Dietary flavonoid fisetin, a phytochemical, has been shown in preclinical studies to inhibit cancer growth through alteration of cell cycle Gupta et al., 2014Haddad et al.,2005Lall et al., 2016. Fisetin also showed its beneficial effects against several diseases. It is a promising novel antioxidant. Fisetin has also been reported as a neuroprotective agent. Fisetin prevents neuroinflammation by suppressing activated neuroinflammatory mediators and gliosis Ahmad et al., 2016. It has also been shown to affect multiple pathways involved in neuronal function in the process of aging Maher, 2009. Fisetin is reported to have anti-inflammatory, and other health beneficial effects. Anti-inflammatory activity of Fisetin exerts via inhibition of Akt, p38 MAPK and NF-κB signaling in the colon tissues of DSS-exposed mice Sahu et al., 2016. It has been reported that, fisetin inhibits LPS)- induced pro-inflammatory cytokine release and also activation of macrophages Kim et al., 2012Liu et al., 2010. Fisetin also showed its antidepressant properties (Sahu et al., 2014).
Curcumin has been shown to act as a cancer chemopreventive and chemotherapeutic agent Chuang, 2000Goel et al., 2008Hatcher et al., 2008Jiao et al., 2008. Curcumin, a polyphenolic compound, has been successfully used for treatment of various diseases such as allergies, arthritis, diabetes, Alzheimer’s disease and other chronic diseases Aggarwal et al., 2007Manolova et al., 2014. It has anti-oxidant potential, ability to inhibit cell signaling affect, various cellular enzymes, cell adhesion and angiogenesis which contribute to its biological activity. Antifibrotic effect of curcumin was described recently by Zhang et al Zhang et al., 2012. Various preclinical, clinical, and animal studies suggest that curcumin is a potential antiproliferative, anti-invasive, and antiangiogenic agent. It also acts as a mediator of chemoresistant, chemopreventive, and therapeutic agent Ara et al., 2016Goel et al.,2008Jiao et al., 2008Rai et al., 2010.
Mesoporous carbon nanoparticles (MCN), with a strong adsorption capacity and easily functionalized surface, have attracted great interest with regard to targeted drug delivery. MCN can also protect the encapsulated agents from degradation during delivery Hartmann et al., 2005Huang et al., 2011Ramanathan et al., 2005Wan et al., 2016Zhao et al., 2012.
In the present study we found that both fisetin and curcumin and MCN treated fisetin demonstrated ameliorative potential in bleomycin induced IPF.
Bleomycin administration is known to damage the lung epithelial cells resulting in lower cell numbers Hay et al., 1991. The results from our colony forming assay in BALF demonstrated a reduction in colonyforming units on bleomycin treatment. The number of colonies increased after treatment with fisetin+MCN and curcumin. The results obtained on treatment with curcumin were statistically significant ( Figure 1 ). Figure 2 showing microscopic examination of colony formation in control, bleomycin treated sample, fisetin, curcumin and fisetin + MCN treated sample. Figure 3A and Figure 3B demonstrating that, all 3 groups (fisetin, curcumin and fisetin + MCN) showed significant reversal of uncontrolled cell migration. Figure 3C showing statistically significant increase in cell migration in bone marrow after bleomycin treatment and significantly reduced in all 3 therapeutic groups. Figure 3D showing statistically significant increase in cell migration in all other groups except curcumin treated group. In summary, Figure 3 ( Figure 3A - Figure 3D ) demonstrating that cellular migration is prevented in primary and secondary lymphoid tissue and regenerated in BALF indicating that cells which are destined to undergo apoptosis in a typical inflammatory situation, may now be differentiated, initiating tissue repair.
While cellular migration in the tissue show slightly better functional profiles, ex-vivo proliferative potential of BALF remains unchanged ( Figure 4A ), indicating that the cells closest to the interstitium are perhaps no longer functionally competent in response to LPS. Figure 4B unequivocally demonstrates true rejuvenation of lung parenchymal cells of mice threaten with MCN treated fisetin and curcumin. This is extremely promising and is corroborated by the results of functional compliance to LPS induced oxidative response in all 3 groups (fisetin, curcumin and fisetin + MCN) compare to post bleomycin treatment only. The lung cells and not the cells that are crossed over into the air space, are critical in initiating post- inflammatory restoration. In comparison with cells of the lung (in-situ or migrated across air space), hematopoietic cells in circulation respond differently to fisetin, MCN treated fisetin and curcumin treatment.
Figure 5C indicates MCN treatment to be ineffective, which is fisetin does not need a vehicle and functionality of circulatory hematopoietic cells is more efficient in the fisetin treated group than in curcumin treatment. Reduction in ROI and RONI in MCN treated fisetin and curcumin treated groups positively indicates that the phytochemicals and their nanocomposites coupled dosing rescues inflammatory damage by inhibiting specific pathways in which inflammation regulatory induced negative feedback is operative.
This is the 1st time to the best of our knowledge that, our data set demonstrates the role of fisetin, curcumin and their nanocomposites coupled forms that may contribute directly to tissue functions in-vivo as well as individual cellular function in ex-vivo inflammatory assay.
The results from NBT assay demonstrated a significant effect of curcumin in BALF, lung and peripheral blood. A significant effect of fisetin was observed in BALF and peripheral blood. Although not significant in the lung tissue, fisetin treatment led to a decrease in superoxide dismutase levels, indicating reduction of oxidative stress. Similarly, the results from NO assay performed on BALF, lung and peripheral blood demonstrated the effects of phytochemicals used in this study on nitric oxide levels. Both fisetin and curcumin reduced NO levels in the samples and the difference was statistically significant. These suggest that, perhaps curcumin and fisetin inhibit the inflammatory or apoptotic pathways. Further, these findings were also supported by the histological analysis of lung samples from fisetin and curcumin treated samples.
Conclusion
In conclusion, we found that, both fisetin and curcumin can be potentially used to improve the pathology of Idiopathic Pulmonary Fibrosis. Further studies examining the molecular pathways affected by these compounds may help in improving their efficacy in treatment of Idiopathic Pulmonary Fibrosis.
Abbreviations
IPF:Idiopathic pulmonary fibrosis
AEC:Alveolar epithelial cell
BALF:Bronchoalveolar lavage fluid
MCN:Mesoporous carbon nanoparticle
PB:Peripheral blood
BM:Bone marrow
TC:Total count
DC:Differential count
IMDM:Iscove’s modified Dulbecco modified medium
CFU:Colony- forming unit
BLEO:Bleomycin
NIN:National Institute of Nutrition
RHMP:Rodent Health Monitoring Programme
ROS:Reactive Oxygen Species
TNF:Tumor Necrosis Factor
TGF:Transforming Growth Factor
PMN:Polymorphonuclear
ECM:Extra Cellular Matrix
NIN:National institute of Nutrition
RHMP:Rodent Health Monitoring Programme
Bleo:Bleomycin
PBS:Phosphate Buffer Saline
NO:Nitric oxide
NBT:Nitro Blue Tetrazolium
References
-
B.B.
Aggarwal,
Y.-J.
Surh,
S.
Shishodia.
The molecular targets and therapeutic uses of curcumin in health and disease. Springer Science & Business Media.
2007;
595
.
-
A.
Ahmad,
T.
Ali,
H.Y.
Park,
H.
Badshah,
S.U.
Rehman,
M.O.
Kim.
Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Molecular Neurobiology.
2016
.
-
R.
Akgedik,
Ş.
Akgedik,
H.
Karamanlı,
S.
Uysal,
B.
Bozkurt,
D.
Ozol,
F.
Armutcu,
Z.
Yıldırım.
Effect of Resveratrol on Treatment of Bleomycin-Induced Pulmonary Fibrosis in Rats. Inflammation.
2012;
35
:
1732-1741
.
-
P.
Anand,
A.B.
Kunnumakkara,
R.A.
Newman,
B.B.
Aggarwal.
Bioavailability of Curcumin: Problems and Promises. Mol Pharmaceutics.
2007;
4
:
807-818
.
-
K.M.
Antoniou,
G.
Margaritopoulos,
F.
Economidou,
N.M.
Siafakas.
Pivotal clinical dilemmas in collagen vascular diseases associated with interstitial lung involvement. European Respiratory Journal.
2009;
33
:
882-896
.
-
K.M.
Antoniou,
A.U.
Wells.
Scleroderma lung disease: evolving understanding in light of newer studies. Current Opinion in Rheumatology.
2008;
20
:
686-691
.
-
S.
Ara,
J.
Mudda,
A.
Lingappa,
P.
Rao.
Research on curcumin: A meta-analysis of potentially malignant disorders. J Can Res Ther.
2016;
12
:
175
.
-
S.O.
Arslan,
M.
Zerin,
H.
Vural,
A.
Coskun.
The effect of melatonin on bleomycin-induced pulmonary fibrosis in rats. J Pineal Res.
2002;
32
:
21-25
.
-
O.I.
Aruoma.
Nutrition and health aspects of free radicals and antioxidants. Food and Chemical Toxicology.
1994;
32
:
671-683
.
-
E.R.
Banerjee,
S.
Kar,
S.
Konsam,
G.
Hore,
S.
Mitra,
S.
Biswas,
A.
Sinha,
N.R.
Jana.
Therapeutic use of fisetin, curcumin, and mesoporous carbon nanoparticle loaded fisetin in bleomycin-induced idiopathic pulmonary fibrosis. Biomedical Research and Therapy.
2015;
2
.
-
P.
Boisseau,
B.
Loubaton.
Nanomedicine, nanotechnology in medicine. omptes Rendus Physique.
2011;
12
:
620-636
.
-
J.
Chen,
J.
Stubbe.
Bleomycins: new methods will allow reinvestigation of old issues. Current Opinion in Chemical Biology.
2004;
8
:
175-181
.
-
S.E.
Chuang.
Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis.
2000;
21
:
331-335
.
-
A.
Goel,
A.B.
Kunnumakkara,
B.B.
Aggarwal.
Curcumin as “Curecumin”: From kitchen to clinic. Biochemical Pharmacology.
2008;
75
:
787-809
.
-
S.C.
Gupta,
A.K.
Tyagi,
P.
Deshmukh-Taskar,
M.
Hinojosa,
S.
Prasad,
B.B.
Aggarwal.
Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Archives of Biochemistry and Biophysics.
2014;
559
:
91-99
.
-
A.Q.
Haddad,
V.
Venkateswaran,
L.
Viswanathan,
S.J.
Teahan,
N.E.
Fleshner,
L.H.
Klotz.
Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis.
2005;
9
:
68-76
.
-
M.
Hartmann,
A.
Vinu,
G.
Chandrasekar.
Adsorption of Vitamin E on Mesoporous Carbon Molecular Sieves. Chemistry of Materials.
2005;
17
:
829-833
.
-
H.
Hatcher,
R.
Planalp,
J.
Cho,
F.M.
Torti,
S.V.
Torti.
Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci.
2008;
65
:
1631-1652
.
-
J.
Hay,
S.
Shahzeidi,
G.
Laurent.
Mechanisms of bleomycin-induced lung damage. Archives of Toxicology.
1991;
65
:
81-94
.
-
H.
Huang,
Q.
Yuan,
J.S.
Shah,
R.D.K.
Misra.
A new family of folate-decorated and carbon nanotube-mediated drug delivery system: Synthesis and drug delivery response. Advanced Drug Delivery Reviews.
2011;
63
:
1332-1339
.
-
Y.
Jiao,
J.
Wilkinson,
X.
Di,
W.
Wang,
H.
Hatcher,
N.D.
Kock,
R.
D’Agostino,
M.A.
Knovich,
F.M.
Torti,
S.V.
Torti.
Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood.
2008;
113
:
462-469
.
-
G.
Kaur,
R.K.
Narang,
G.
Rath,
A.K.
Goyal.
Advances in Pulmonary Delivery of Nanoparticles. Artificial.
2011;
Cells
:
Blood Substitutes, and Biotechnology 40, 75-96
.
-
S.-C.
Kim,
S.-H.
Kang,
S.-J.
Jeong,
S.-H.
Kim,
H.S.
Ko,
S.-H.
Kim.
Inhibition of c-Jun N-terminal kinase and nuclear factor κ B pathways mediates fisetin-exerted antiinflammatory activity in lipopolysccharide-treated RAW264.7 cells. Immunopharmacology and Immunotoxicology.
2012;
34
:
645-650
.
-
P.
Kumaravelu,
D.P.
Dakshinamoorthy,
S.
Subramaniam,
H.
Devaraj,
N.S.
Devaraj.
Effect of eugenol on drugmetabolizing enzymes of carbon tetrachloride-intoxicated rat liver. Biochemical Pharmacology.
1995;
49
:
1703-1707
.
-
R.K.
Lall,
V.M.
Adhami,
H.
Mukhtar.
Dietary flavonoid fisetin for cancer prevention and treatment. Molecular Nutrition & Food Research.
2016;
60
:
1396-1405
.
-
S.-H.
Liu,
C.-H.
Lin,
S.-K.
Hung,
J.-H.
Chou,
C.-W.
Chi,
S.-L.
Fu.
Fisetin Inhibits Lipopolysaccharide-Induced Macrophage Activation and Dendritic Cell Maturation. J Agric Food Chem.
2010;
58
:
10831-10839
.
-
P.
Maher.
Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes & Nutrition.
2009;
4
:
297-307
.
-
T.M.
Maher,
A.U.
Wells,
G.J.
Laurent.
Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms?. European Respiratory Journal.
2007;
30
:
835-839
.
-
Y.
Manolova,
V.
Deneva,
L.
Antonov,
E.
Drakalska,
D.
Momekova,
N.
Lambov.
The effect of the water on the curcumin tautomerism: A quantitative approach. pectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.
2014;
132
:
815-820
.
-
A.
Moeller,
K.
Ask,
D.
Warburton,
J.
Gauldie,
M.
Kolb.
The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis?. The International Journal of Biochemistry & Cell Biology.
2008;
40
:
362-382
.
-
R.
Mohamud,
S.D.
Xiang,
C.
Selomulya,
J.M.
Rolland,
R.E.
O’Hehir,
C.L.
Hardy,
M.
Plebanski.
The effects of engineered nanoparticles on pulmonary immune homeostasis. Drug Metabolism Reviews.
2013;
46
:
176-190
.
-
B.
Rai,
J.
Kaur,
R.
Jacobs,
J.
Singh.
Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci.
2010;
52
:
251-256
.
-
T.
Ramanathan,
F.T.
Fisher,
R.S.
Ruoff,
L.C.
Brinson.
Amino-Functionalized Carbon Nanotubes for Binding to Polymers and Biological Systems. Chemistry of Materials.
2005;
17
:
1290-1295
.
-
B.D.
Sahu,
A.K.
Kalvala,
M.
Koneru,
J.
Mahesh Kumar,
M.
Kuncha,
S.S.
Rachamalla,
R.
Sistla.
Ameliorative Effect of Fisetin on Cisplatin-Induced Nephrotoxicity in Rats via Modulation of NF-κB Activation and Antioxidant Defence. PLoS ONE.
2014;
9
:
e105070
.
-
B.D.
Sahu,
J.M.
Kumar,
R.
Sistla.
Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of NF-κB signaling. The Journal of Nutritional Biochemistry.
2016;
28
:
171-182
.
-
K.
Shi,
J.
Jiang,
T.
Ma,
J.
Xie,
L.
Duan,
R.
Chen,
P.
Song,
Z.
Yu,
C.
Liu,
Q.
Zhu.
Dexamethasone attenuates bleomycin-induced lung fibrosis in mice through TGF-β, Smad3 and JAK-STAT pathway. International journal of clinical and experimental medicine.
2014;
7
:
2645
.
-
N.W.
Todd,
I.G.
Luzina,
S.P.
Atamas.
Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis & Tissue Repair.
2012;
5
:
11
.
-
R.
Verma,
L.
Kushwah,
D.
Gohel,
M.
Patel,
T.
Marvania,
S.
Balakrishnan.
Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulmonary Medicine.
2013;
2013
:
1-10
.
-
D.M.
Walters,
H.-Y.
Cho,
S.R.
Kleeberger.
Oxidative Stress and Antioxidants in the Pathogenesis of Pulmonary Fibrosis: A Potential Role for Nrf2. Antioxidants & Redox Signaling.
2008;
10
:
321-332
.
-
L.
Wan,
J.
Jiao,
Y.
Cui,
J.
Guo,
N.
Han,
D.
Di,
D.
Chang,
P.
Wang,
T.
Jiang,
S.
Wang.
Hyaluronic acid modified mesoporous carbon nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanotechnology.
2016;
27
:
135102
.
-
H.-D.
Wang,
M.
Yamaya,
S.
Okinaga,
Y.-X.
Jia,
M.
Kamanaka,
H.
Takahashi,
L.-Y.
Guo,
T.
Ohrui,
H.
Sasaki.
Bilirubin Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Rats. Am J Respir Crit Care Med.
2002;
165
:
406-411
.
-
M.S.
Wilson,
T.A.
Wynn.
Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol.
2009;
2
:
103-121
.
-
S.-S.
Zhang,
Z.-J.
Gong,
W.-H.
Li,
X.
Wang,
T.-Y.
Ling.
Antifibrotic effect of curcumin in TGF-β1-induced myofibroblasts from human oral mucosa. Asian Pacific Journal of Cancer Prevention.
2012;
13
:
289-294
.
-
P.
Zhao,
L.
Wang,
C.
Sun,
T.
Jiang,
J.
Zhang,
Q.
Zhang,
J.
Sun,
Y.
Deng,
S.
Wang.
Uniform mesoporous carbon as a carrier for poorly water soluble drug and its cytotoxicity study. European Journal of Pharmaceutics and Biopharmaceutics.
2012;
80
:
535-543
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 07 (2016)
Page No.: 707-722
Published on: 2016-07-26
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5753 times
- Download PDF downloaded - 1956 times
- View Article downloaded - 12 times
 Biomedpress
Biomedpress