Anemia in children: A review
- School of Medical Sciences, School of Dental Sciences, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
- Hospital Pakar Universiti Sains Malaysia, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
- School of Dental Sciences, School of Dental Sciences, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
- School of Health Sciences, School of Dental Sciences, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
Abstract
Anemia represents a significant global health challenge, particularly among children. It arises from multiple causes. The impact of anemia is substantial, leading to fatigue and weakness, which can restrict physical activity. In children, it can also impair cognitive development, affecting learning ability and concentration. This underscores its profound influence on overall quality of life and developmental outcomes. Effective control of anemia demands a comprehensive approach. Early detection and timely intervention are critical to mitigating the long-term consequences of anemia on individuals and communities. Reducing anemia, especially in children, is cost-effective and yields substantial health benefits. Therefore, to achieve prevention, it is important to raise awareness in the community about anemia and its complications, because mild or moderate anemia, if unrecognized and untreated, can progress to severe and life-threatening stages. Accordingly, this review offers a comprehensive overview of anemia, encompassing inherited and acquired causes. It includes prevalence rates, regional studies, and detailed tables. Finally, the integration of scientific references and study findings confers validity, rendering it a supported and informative review.
Introduction
Anemia remains a major public health problem. Approximately 1.62 billion individuals worldwide are affected, with a particularly high prevalence among children 1. According to the World Health Organization (WHO), anemia in adults is defined as a hemoglobin (Hb) concentration of <12 g/dL in women and <13 g/dL in men. In children, the WHO Hb cut-off values vary by age, as summarized in
Hemoglobin level based on severity of anemia in children
| Anemia (g/dL) | ||||
|---|---|---|---|---|
| Age of children | Normal | Mild | Moderate | Severe |
| 5 years of age and below | 11.0 | 10.0-10.9 | 7.0-9.90 | < 7 |
| 5-11 years | 11.5 | 11.0-11.4 | 8.0-10.9 | < 8.0 |
| 12-14 years | 12.0 | 11.0-11.9 | 8.0-10.9 | < 8.0 |
| 15 years of age and above | >12.0 | 10.0-10.9 | 7.0-9.9 | > 7.0 |
Methods
This review was conducted to provide a comprehensive overview of anemia in children, focusing on its prevalence, causes, diagnosis, and management. A structured literature search was performed across the following electronic databases: PubMed, Scopus, and Google Scholar, covering the period from 1993 to 2023. The search terms used included combinations of the following keywords: "anemia", "pediatric anemia", "children", "iron deficiency anemia", "nutritional anemia", "hemoglobin", and "pediatric hematology", using Boolean operators (AND, OR) to refine the search.
Inclusion criteria were peer-reviewed original research articles, reviews, and clinical guidelines written in English that addressed the epidemiology, etiology, diagnosis, treatment, or prevention of anemia. Exclusion criteria included non-English articles and studies focusing exclusively on adults or on other hematologic disorders not primarily related to anemia. The findings from the selected literature were synthesized thematically, focusing on major areas such as prevalence, etiology, clinical presentation, treatment, and preventive strategies.
Inherited anemia
Hereditary disorders of red blood cells (RBCs) arise from mutations or deletions in specific genes and can result in accelerated RBC destruction and anemia due to decreased hemoglobin (Hb) levels. These inherited RBC disorders include defects in hemoglobin (hemoglobinopathies), the RBC membrane (membranopathies), and RBC enzymes (enzymopathies) 4.
Hemoglobinopathy
Hemoglobinopathies constitute one of the most prevalent genetic disorders among children worldwide. They are broadly classified into two groups: thalassemias and structural hemoglobin (Hb) variants (abnormal hemoglobins) 5.
Thalassemias are subdivided into β-thalassemia and α-thalassemia, whereas common Hb variants include HbS, HbE, and HbC 6,7. Thalassemia affects both sexes and is particularly prevalent in the Mediterranean basin, Africa, the Middle East, the Indian sub-continent, and South-East Asia. Approximately 80 million individuals are carriers and 300 000–400 000 children are born annually with severe phenotypes. Recent investigations have concentrated on the prevalence, molecular genetics, and clinical consequences of α- and β-thalassemia 8-9.
For example, neonatal screening of 1438 infants in Hainan Province, China (2020–2021), identified 1024 thalassemia carriers, most of whom had α-thalassemia; comparable prevalence figures have been reported from Thailand and Malaysia 10,11,12,13,14. In South-East Asia, HbE/β-thalassemia represents a frequent and clinically important phenotype that often necessitates regular transfusion therapy. Owing to increasing migration and inter-marriage, its prevalence is expanding worldwide 15,16,17. Public-health interventions—including prenatal and premarital screening, population education, and genetic counselling—have demonstrated efficacy in lowering disease burden 17.
Structural Hb variants arise from mutations, deletions, substitutions, stop-codon read-through (antitermination), or aberrant post-translational modifications of the globin chain 18. As for thalassemia, the prevalence of Hb variants is augmented by consanguineous marriage and population migration. The most prevalent variants are HbE, HbC, and HbS 19,20,21,22,23.
HbE results from a single-nucleotide substitution that replaces glutamic acid with lysine at codon 26 of the β-globin gene 19. A majority of affected children in Malaysia harbour this variant 24,25,26. In one cohort, 2 of 29 paediatric patients with HbE/β-thalassemia developed thromboembolic events 27, which may be attributable to genetic predisposition, haemostatic abnormalities, or hepatic dysfunction 28.
HbC arises from the substitution of glutamic acid by lysine at codon 6 of the β-globin chain 20. It is highly prevalent in West Africa, particularly in Ghana 20. Most heterozygous carriers remain asymptomatic; however, homozygotes may present with mild anaemia, jaundice, or splenomegaly 21.
HbS, the hallmark of sickle cell disease, is produced by the replacement of glutamic acid with valine at codon 6 of the β-globin chain 22. This amino-acid change reduces the molecule’s anionic charge and solubility under de-oxygenated conditions, promoting HbS polymerisation, erythrocyte sickling, chronic haemolysis, and recurrent vaso-occlusive crises 23. In 2010 an estimated 305 000 infants were born with sickle cell disease worldwide, and the majority of related childhood deaths occurred in low- and middle-income countries 31.
In Sudan, HbAS and HbSS were reported in 11.3 % and 3.5 %, respectively, of children aged 0–18 years 32. HbSS prevalence is particularly high in Africa, contributing substantially to childhood morbidity and mortality. Carrier frequencies (HbAS) often exceed 20 %, whereas the prevalence of affected individuals (HbSS) is at least 2 % 35. Among 102 Nigerian children aged 7 months–17 years, 97.1 % had HbSS, whereas 2.9 % had HbSC. In Cameroon, screening of 703 infants revealed HbSS in 0.7 %, HbS/β+-thalassemia in 0.6 %, and HbAS in 16.8 % 36. Comparable figures have been documented in the Congo, where 1.4 % of 204 neonates carried HbSS and 16.9 % carried HbAS 37,38. These data from Sudan, Nigeria, Cameroon, and the Congo underline the urgency of implementing universal newborn screening and early-intervention programmes to mitigate sickle cell-related morbidity and mortality.
Membranopathy
Hereditary spherocytosis (HS) is an inherited membranopathy caused by defects in red blood cell membrane proteins, which can lead to hemolytic anemia. HS is characterized by abnormally spherical red blood cells (spherocytes) that are more fragile than normal disc-shaped erythrocytes and therefore have a shortened lifespan. In children, HS can be detected by the presence of spherocytes on a peripheral blood smear 39. There is an association between HS and anemia, leading to several clinical manifestations such as jaundice, risk of gallstones, splenomegaly, functional hyposplenia, and reticulocytosis 40. The severity of anemia in HS varies, ranging from mild to severe 41.
The bar chart in Figure 1 summarizes the clinical features of HS reported in five pediatric studies comprising 137 patients published between 1991 and 2021 42,43,44,45,46. Most pediatric cases present with anemia, followed by splenomegaly, transfusion requirement, jaundice, and neonatal jaundice. HS is more frequent in Northern Europeans than in Southeast Asians. The number of pediatric patients with gallstones was relatively low. Nevertheless, the current incidence and prevalence of HS in Malaysia remain unknown because of the limited number of publications 41,42,43,44,45,46.
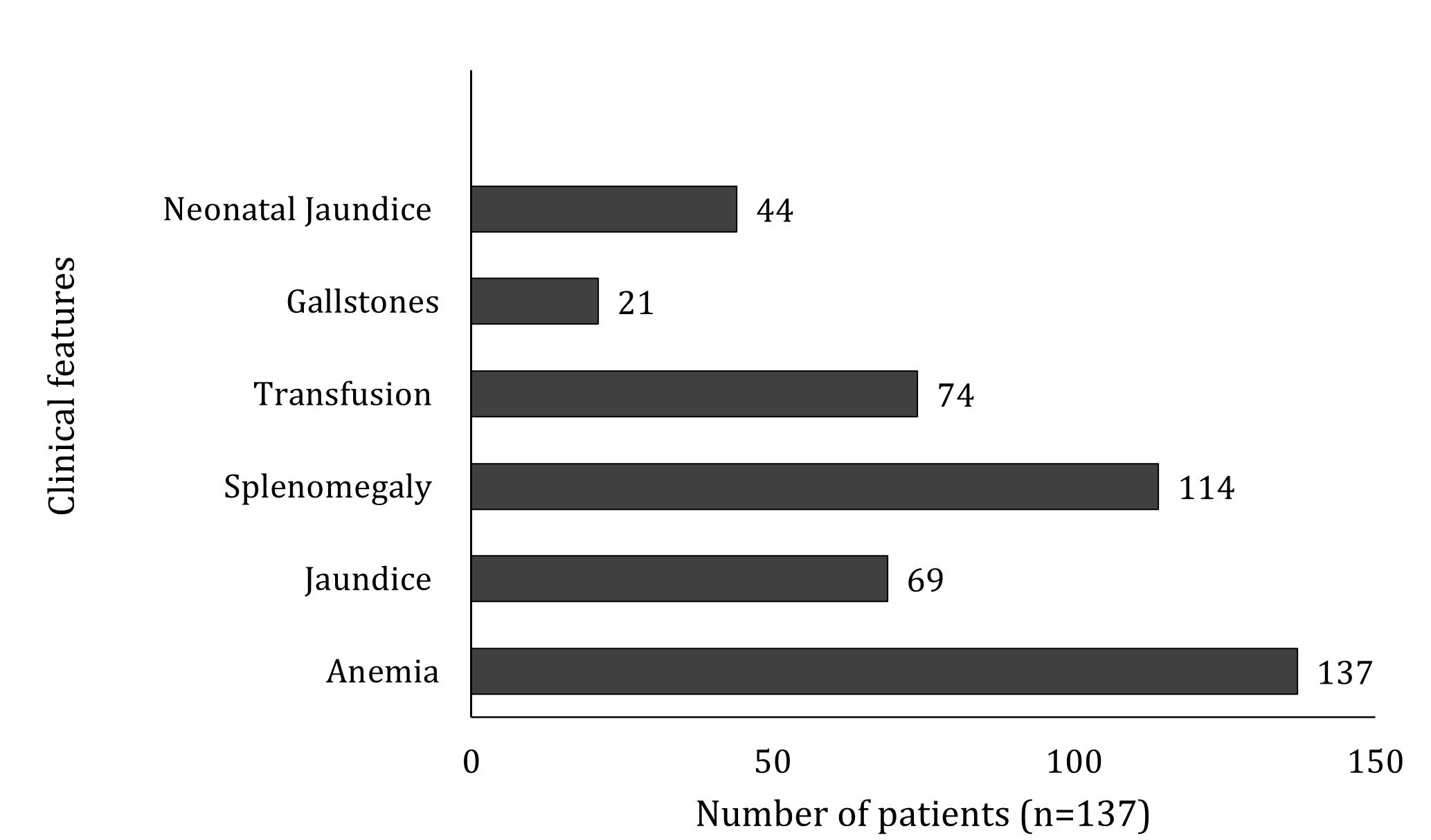
Clinical features of hereditary spherocytosis in 137 patients. The bar chart summarizes the frequency of common clinical manifestations reported across five pediatric studies. Abbreviation: HS = Hereditary spherocytosis.
Enzymopathy
Moreover, glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzymopathy worldwide, particularly in children. The G6PD gene is located on the X chromosome, specifically in the subtelomeric region (Xq28) 47. The enzyme maintains cellular homeostasis by generating reduced nicotinamide adenine dinucleotide phosphate (NADPH), thereby protecting erythrocytes from oxidative damage and premature destruction; it also functions as a housekeeping enzyme by limiting injury caused by reactive oxygen species (ROS). Because the G6PD gene is X-linked, males are more frequently affected than females: a hemizygous male requires only one defective allele to manifest G6PD deficiency, whereas females possess two copies of the gene and may be homozygous normal, heterozygous (intermediate), or homozygous deficient 48,49. Individuals with G6PD deficiency may develop acute hemolytic anemia following exposure to oxidative foods, medications, or chemical agents—such as fava beans, antimalarial drugs, and aspirin 50,51,52.
The global prevalence of G6PD deficiency is approximately 4.9 % 53. A study by Ainoon et al. 54 described a boy with a history of recurrent fever, pallor, and dark-colored urine after exposure to mosquito repellent and traditional Chinese herbs; laboratory evaluation confirmed hemolytic anemia, and molecular analysis identified a 24-bp deletion of nucleotides 953–976 in exon 9 of the G6PD gene 53,54. A prevalence of 14.4 % was reported among 118 children aged 2–5 years 48. The predominance in males is consistent with the requirement for biallelic mutations in females to express the phenotype 55,56. Consequently, neonatal screening in high-prevalence regions, parental education on the avoidance of oxidative triggers, and genetic counseling are recommended to prevent hemolytic crises and support at-risk families.
Acquired anemia
Acquired anemia may arise from iron deficiency, dietary insufficiency, inflammation, menorrhagia, chronic disorders, and infectious diseases 57,58. Most conditions associated with anemia can be classified into three major groups: anemia of chronic disease, infectious anemia, and nutritional deficiency anemia 59.
Anemia of chronic disease (ACD), also referred to as anemia of chronic inflammation, is one of the most common causes of acquired anemia 60,61,62. Its pathogenesis is mediated by cytokines—including interferon (IFN), interleukins (IL), tumor necrosis factor (TNF), and hepcidin—released after activation of T lymphocytes and macrophages in malignancy and autoimmune disorders. These mediators down-regulate erythropoietin receptors through sustained release of pro-inflammatory cytokines and the generation of reactive oxygen species 63. Consequently, interferon-γ (IFN-γ) exerts direct toxicity on erythroid progenitors, thereby worsening the severity of ACD 64. A concomitant reduction in circulating erythropoietin further aggravates the anemia 65. Inflammatory signaling depletes functional iron stores, leading to a fall in hemoglobin concentration 66. Disruption of iron homeostasis and a shortened red-cell lifespan further contribute to the pathophysiology of ACD 67. Accordingly, the peripheral blood film is typically normocytic–normochromic, although microcytosis can emerge in advanced disease 62,68.
Chronic kidney disease (CKD) is a major cause of ACD because renal impairment limits erythropoietin synthesis. CKD is defined as structural or functional kidney damage with a glomerular filtration rate (GFR) below 60 mL/min/1.73 m² 69. If left untreated, CKD markedly increases the risk of adverse events, including stroke 70. CKD is stratified into five stages of renal dysfunction, which are often asymptomatic in the early phases. Previous studies report a high prevalence of severe normocytic anemia in older adults with stage 5 CKD 71. The prevalence of CKD in children has risen steadily since the 1980s, paralleling an increase in anemia 72. In paediatric patients, CKD-related anemia adversely affects growth and quality of life 72,81. Anemic CKD further heightens the risk of cardiovascular complications 73. CKD-associated anemia is characterized by reduced hemoglobin concentration and a shortened erythrocyte lifespan 82. In the Korean KNOW-PedCKD cohort, Lee et al. (2019) demonstrated that school-aged children with stage 4 CKD had a 31.4 % prevalence of anemia, significantly higher than in earlier stages. Similarly, >20 % of paediatric patients reach end-stage renal disease (ESRD) with concomitant anemia 74,75. Salman et al. (2016) found an anemia prevalence of 83.3 % among female CKD patients in north-eastern Peninsular Malaysia; 79 % had severe and 34.4 % had moderate anemia. Declines in Hb, MCH, and MCHC correlated with worsening renal function in these patients 76. The primary mechanism is inadequate erythropoietin (EPO) production by the diseased kidney, which impairs erythropoiesis 77. Ageing further diminishes EPO responsiveness, exacerbating anemia 78.
Infectious diseases contribute to anemia by disrupting iron metabolism and altering systemic iron balance. In children, the most common infectious contributors are parasitic infections—particularly soil-transmitted helminths (STH) and malaria 79. STH ova mature in soil, facilitating transmission of intestinal worms that disproportionately affect school-aged children. Risk factors include barefoot outdoor play, inadequate hand hygiene, and poor nail care, which enable larval penetration. Prevalence studies of STH-related anemia—principally involving hookworm (Necator americanus, Ancylostoma duodenale), Ascaris lumbricoides, and Trichuris trichiura—are summarized in
Prevalence of soil transmitted helminth (STH) associated with anemia among children based on many study
| No | References | Country | Study population | Sample size | Type of study | Laboratory Technique | Prevalence (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | STH | Hookworm | Roundworm | Whipworm | |||||||
| 1 | Malaysia | School children | 254 | cross sectional | Kato-Katz | 41 | 93.7 | 3.9 | 47.6 | 84.6 | |
| 2 | Malaysia | School children | 148 | cross sectional | Formalin ether concentration | 37.8 | 37.20 | 8.70 | 44.90 | 46.40 | |
| 3 | Indonesia | School children | 82 | cross sectional | Kato-Katz | 2.40 | 7.30 | non detected | 3.70 | 2.40 | |
| 4 | Indonesia | Preschool children | 393 | cross sectional | Kato-Katz | 60.30 | 58.80 | 9.2 | 47.40 | 36.80 | |
| 5 | Thailand | School children | 375 | cross sectional | Formalin ether concentration | 6.40 | 47.70 | 0.50 | 13.30 | 16.30 | |
Malaria is another major infection underlying pediatric anemia. Disease severity correlates with transmission intensity, vector density, longevity, biting behaviour, and vector competence. Plasmodium falciparum and P. vivax predominate and are highly prevalent in Asia and sub-Saharan Africa 85. Between 2000 and 2019, 279 paediatric cases of malaria-associated anemia were reported (
Summary for malaria cases that is associated with anemia condition
| No | Country | Years of study | No sample | Age (years old) | Species | Anemic (Hb level in g/dL) | Severe | Moderate | Mild | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Malaysia | 2009 | 220 | <15 | 30 (<10) | NS | NS | NS | ||
| 2 | Vietnam | 2012-2019 | 47 | <15 | 47 (<11) | 3 | 44 | 0 | ||
| 3 | Laos | 2010-2011 | 319 | 2.5-10 | 92 (11.5) | 2 | 49 | 41 | ||
| 4 | Thailand-Myanmar Border | 2000-2016 | 926 | <15 | 110 (<5) | 110 | NS | NS |
Nutrient deficiencies are a common cause of anemia, particularly in children of both sexes. Dietary imbalances increase the risk of the most prevalent micronutrient deficiencies, namely vitamin B12, folic acid and, in particular, iron deficiency 89. Prolonged depletion of vitamin B12, folic acid or iron stores leads to megaloblastic anemia, iron-deficiency anemia, or to the simultaneous occurrence of both 90.
Adequate iron intake is essential for fetal and infant development 91,92. Iron deficiency is the most widespread nutritional inadequacy associated with anemia; it affects more than 30 % of the global population and is the leading contributor to the condition 1,93,94. Malnutrition significantly lowers haemoglobin, mean corpuscular haemoglobin (MCH) and mean corpuscular volume (MCV) in children under five years of age. The prevalence of iron-deficiency anemia (IDA) and iron deficiency (ID) is about 40 % among pre-school children in low- and middle-income countries 95. Among five-year-old children, the prevalence of IDA is 18.6 % (55/295). In Malaysia, approximately 4 % of primary-school children are anemic 96,97. Long-term IDA adversely affects neurodevelopment, including neurotransmitter metabolism and memory function 98.
These findings underscore the substantial burden of anemia in paediatric populations, particularly in endemic regions where repeated infections such as malaria and co-existing nutritional deficiencies further aggravate anemia severity.
Conclusion
Addressing pediatric anemia requires an integrated approach that combines clinical interventions, public-health initiatives, and ongoing research. For inherited disorders such as HbE/β-thalassemia, expansion of newborn screening, provision of genetic counseling, and investigation of genetic modifiers are critical for early detection and optimum management. In cases of acquired anemia, priority should be given to strengthening nutrition programmes, implementing infection-control measures, and increasing public awareness. Enhanced global collaboration among researchers, policy-makers, and public-health agencies is essential to develop sustainable solutions and to mitigate the worldwide burden of childhood anemia. Nevertheless, substantial knowledge gaps persist, particularly regarding the contribution of genetic modifiers to inherited anemias and the optimisation of targeted therapies. Additional investigations into epigenetic determinants of disease severity, innovative pharmacotherapies, and affordable gene-based treatments are required to transform the management of anemia.
Abbreviations
ACD: Anemia of Chronic Disease; CKD: Chronic Kidney Disease; EPO: Erythropoietin; ESRD: End-Stage Renal Disease; FRGS: Fundamental Research Grant Scheme; GFR: Glomerular Filtration Rate; G6PD: Glucose-6-Phosphate Dehydrogenase; Hb: Hemoglobin; HS: Hereditary Spherocytosis; IDA: Iron-Deficiency Anemia; IFN: Interferon; IFN-γ: Interferon-gamma; IL: Interleukins; MCH: Mean Corpuscular Hemoglobin; MCHC: Mean Corpuscular Hemoglobin Concentration; MCV: Mean Corpuscular Volume; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; RBCs: Red Blood Cells; ROS: Reactive Oxygen Species; STH: Soil-Transmitted Helminths; TNF: Tumor Necrosis Factor; WHO: World Health Organization
Acknowledgments
This study was supported by Fundamental Research Grant Scheme (FRGS); 203.PPSP.6171251.
Author’s contributions
Siti Nur Nabeela A'ifah Mohammad and Zefarina Zulkafli contributed to conceptualization; Wan Suriana Wan Ab Rahman and Mohd Nazri Hassan were involved in methodology; Siti Nur Nabeela A'ifah Mohammad and Zefarina Zulkafli contributed to writing original draft preparation; Hisham Atan Edinur, Wan Suriana Wan Ab Rahman and Mohd Nazri Hassanwere involved in writing, review and editing; and all authors have read, approved the final manuscript and agreed to the published.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

