Development and characterization of Melittin-IL-24 fusion protein with synergistically enhanced anti-tumor activity
- Centre for Applied Molecular Biology (CAMB), 87-West canal, Bank Road, University of the Punjab, Lahore-53700, Pakistan
- University Institute of Medical Lab Technology, Faculty of Allied Health Sciences. The University of Lahore -54590 Pakistan
Abstract
Introduction: Targeted cancer therapy using recombinant fusion proteins offers a novel and precise approach to addressing limitations associated with conventional treatments, such as nonspecific toxicity and drug resistance. Fusion proteins are designed to synergistically incorporate multiple protein domains, thereby enhancing bioactivity and creating novel functional combinations with broad applications. In this study, we report the innovative design and evaluation of a fusion protein that combines interleukin-24 (IL-24), a tumor-suppressive cytokine, with melittin, a potent cell-lytic peptide derived from honey-bee venom.
Methods: This combination synergistically unites the pro-apoptotic properties of IL-24 with the membrane-disrupting activity of melittin to increase cytotoxicity against cancer cells. The Melittin-IL-24 construct was expressed in Escherichia coli BL21(DE3) and induced with IPTG.
Results: After affinity chromatography, we obtained ~100 mg of > 97 %-pure fusion protein per liter of culture, as verified by SDS-PAGE, Western blotting, and HPLC. MTT assays showed significant cytotoxicity of Melittin-IL-24 toward MCF-7 breast-cancer cells (IC₅₀ = 52 µg/mL), whereas IL-24 alone exhibited an IC₅₀ of 66 µg/mL. Both proteins were far less toxic to normal MCF-10F mammary epithelial cells.
Conclusion: By combining the apoptotic effects of IL-24 with the cell-lytic properties of melittin, Melittin-IL-24 demonstrates enhanced therapeutic potential and may represent a promising strategy for targeted cancer therapy.
Introduction
Cancer is a complex disease driven by aberrant genetic and epigenetic mechanisms that disrupt fundamental cellular processes such as resistance to apoptosis and uncontrolled proliferation, and therefore represents a substantial global health threat12. Severe side effects associated with conventional chemotherapy underscore the urgent need for novel anticancer therapies34. Natural products, including biotoxins, are increasingly investigated for their therapeutic potential5. Breast cancer, the second leading cause of cancer-related death in women worldwide, remains challenging to treat because of its heterogeneity67. In 2012, approximately two million women were diagnosed with breast cancer, resulting in more than half a million deaths8. Although chemotherapy, radiotherapy, immunotherapy, and targeted therapy are pivotal, each has limitations, highlighting the necessity for innovative approaches910.
Gene-fusion techniques, essential in both basic research and biopharmaceutical development, have revolutionized therapeutic-protein engineering11. Fusion-protein technology has yielded molecules for diverse applications, including cancer treatment. Despite competition from monoclonal antibodies, fusion proteins offer distinct advantages: they can trigger immune responses, induce tumor regression, and deliver cytotoxic payloads directly to cancer cells. Moreover, fusion proteins are often more effective and stable than their natural counterparts1213. By providing multivalent or multispecific binding, they increase target specificity and affinity, thereby enhancing efficacy against cells that express multiple receptors and reducing off-target effects14. Fusion to half-life–extending partners—such as Fc regions or albumin—significantly prolongs circulation time, decreases proteolytic degradation, and lowers dosing frequency15. The versatility of fusion-protein design thus enables tailored strategies for many diseases, particularly cancer.
Interleukin-24 (IL-24) is a cytokine in the IL-10 family that regulates immune responses and induces apoptosis in cancer cells16. IL-24 acts through several mechanisms—apoptosis induction, angiogenesis inhibition, and immune-surveillance enhancement—making it a promising antitumor agent, especially for breast cancer17. In breast cancer, IL-24 selectively induces apoptosis in malignant cells while sparing normal cells, underlining its potential for targeted therapy1819. Activation of the endoplasmic-reticulum stress pathway and generation of reactive oxygen species contribute to this selectivity20. IL-24 not only suppresses tumor growth but also potentiates standard chemotherapeutics2122, positioning it as a valuable adjunct in breast-cancer therapy.
Melittin, the principal component of honey-bee venom, possesses potent cell-lytic and anticancer activities. Its primary mechanism is membrane disruption that triggers apoptosis and inhibits proliferation23. Melittin can selectively target cancer cells, thereby improving the efficacy of conventional therapies. For example, Orsolić showed that melittin inhibits metastasis and induces apoptosis in breast cancer by modulating pro- and anti-apoptotic gene expression24. Down-regulation of matrix metalloproteinases by melittin also reduces tumor invasiveness and metastasis25.
Previously, we computationally engineered a bifunctional fusion protein, melittin-IL-24, which combines melittin’s lytic activity with IL-24’s antitumor properties. In-silico studies suggested that melittin-IL-24 could effectively inhibit breast-cancer cell growth26; however, its potential in cancer immunotherapy remains unexplored.
The main objective of the present study was to express recombinant melittin-IL-24 in , purify the protein by affinity chromatography, and evaluate its biological activity against MCF-7 breast-cancer cells.
Methods
Designing of fusion protein construct
To design the Melittin-IL-24 fusion protein construct, the FASTA nucleotide sequences of the human interleukin-24 gene and the melittin peptide were downloaded from the NCBI database. The nucleotide sequence encoding the cell-killing peptide melittin (GIGAVLKVLTTGLPALISWIKRKRQQ) was fused to the sequence encoding interleukin-24 via a rigid linker (EAAAKEAAAKEAAAK)27. To facilitate purification, the N terminus of the fusion gene carries a 6×His tag. The Swiss-Model web server was used to predict the three-dimensional structure of the fusion protein and to confirm that each domain can fold independently. analysis of the construct was performed, and the predicted results have been reported previously by our lab26. Finally, the melittin-IL-24 gene was synthesized by Twist Bioscience (USA) for subsequent expression studies.
Melittin-IL-24 gene cloning
The melittin-IL-24 gene, synthesized and initially cloned into pUC57 with NdeI (5′) and XhoI (3′), was subcloned into pET-28 under the control of the T7 promoter. Successful cloning was confirmed by PCR amplification followed by double digestion with NdeI and XhoI. The recombinant plasmid was transformed into BL21(DE3) using the CaCl method.
Expression of the melittin-IL-24 fusion gene
Positive transformants were grown overnight at 37 C in LB containing the appropriate antibiotics, then inoculated into 2.5-L baffled flasks with 500 mL M9 medium plus antibiotics and salts. After 6–8 h, when the culture reached the desired OD, expression was induced with 1 mM IPTG. The culture was incubated further, cells were harvested at 5 000 rpm, and the pellet was stored at –20 C.
Cell disruption
Cell pellets were resuspended (1 g pellet / 10 mL) in lysis buffer (50 mM Tris-HCl, pH 8.0; 5 mM EDTA; 1 mM PMSF) and disrupted with a French press at 1.5 kbar. After centrifugation, the supernatant was discarded and the inclusion bodies (IBs) were washed extensively to remove debris.
Solubilization and refolding
IBs were solubilized in 6 M guanidine-HCl with gentle stirring for 30 min at room temperature, followed by centrifugation at 8 000 × g, 4 C for 20 min. Protein concentration was determined by the Bradford assay. The denatured protein was slowly diluted (1:20) into refolding buffer (50 mM Tris-HCl, pH 8.0; 0.4 M L-arginine; 1 mM EDTA; 2 mM GSH; 0.2 mM GSSG) at 4 C with gentle stirring and incubated overnight. Refolding efficiency was estimated at 60–70 % based on SDS-PAGE band intensity and bioactivity recovery28.
Melittin-IL-24 fusion protein purification
The refolded solution was filtered through a 0.45 µm nitrocellulose membrane and concentrated/desalted on a Millipore tangential flow system equipped with a 10 kDa cutoff filter. The protein was then purified by Ni-NTA affinity chromatography. The column was equilibrated with buffer A (20 mM sodium phosphate, 0.5 M NaCl, pH 7.4), washed with buffer B (20 mM imidazole, 20 mM sodium phosphate, 0.5 M NaCl, pH 7.4), and eluted with imidazole-containing elution buffer (pH 7.4). Eluted fractions were pooled for further analysis.
SDS-PAGE and Western blot
Purity was analyzed on 12 % SDS-PAGE following the Laemmli method29. Samples (protein:sample buffer = 1:1) were boiled for 10 min and electrophoresed at 110 V for 100 min. Gels were stained with Coomassie Blue. For Western blotting, proteins were transferred to a PVDF membrane, blocked with 5 % skim milk overnight, incubated with mouse anti-6×His primary antibody (1:1000, 1 h), washed, then incubated with goat anti-mouse HRP-conjugated secondary antibody. Detection was performed with an NBT/BCIP substrate.
HPLC analysis
Protein purity was also assessed by RP-HPLC on a C18 column (25 cm × 4.6 mm, 5 µm, 300 Å) using a Shimadzu LC-20 system. After column equilibration with 0.1 % TFA/10 % acetonitrile, 200 µL of diluted sample was injected. A linear gradient to 80 % 0.1 % TFA/85 % acetonitrile over 25 min, then to 95 % over 5 min, was run at 1 mL min.
Cytotoxicity assay
Cytotoxic effects of melittin-IL-24 and IL-24 were evaluated on MCF-7 (ATCC HTB-26) and non-tumorigenic MCF-10F (ATCC CRL-10318) cells by MTT assay. Cells (1 × 10 cells well) were seeded in DMEM with 10 % FBS, penicillin/streptomycin, and incubated 72 h at 37 C, 5 % CO. Triplicate wells were treated with 10–140 µg mL protein; untreated cells served as controls. After 24 h, 20 µL of 5 mg mL MTT was added and incubated 4 h. Formazan crystals were dissolved in 100 µL DMSO and absorbance was read at 570 nm (reference 630 nm).
Percentage cell viability (%) = (A test – A test) × 100 ⁄ (A control – A control)
Statistical analysis
Statistical analysis was performed with GraphPad Prism. Means ± SD were calculated from triplicate experiments. One-way ANOVA (p ≤ 0.05) followed by Tukey’s HSD test was used to compare cytotoxicity of IL-24 and melittin-IL-24 toward MCF-7 and MCF-10F cells.
Results
Melittin–IL-24 fusion construct
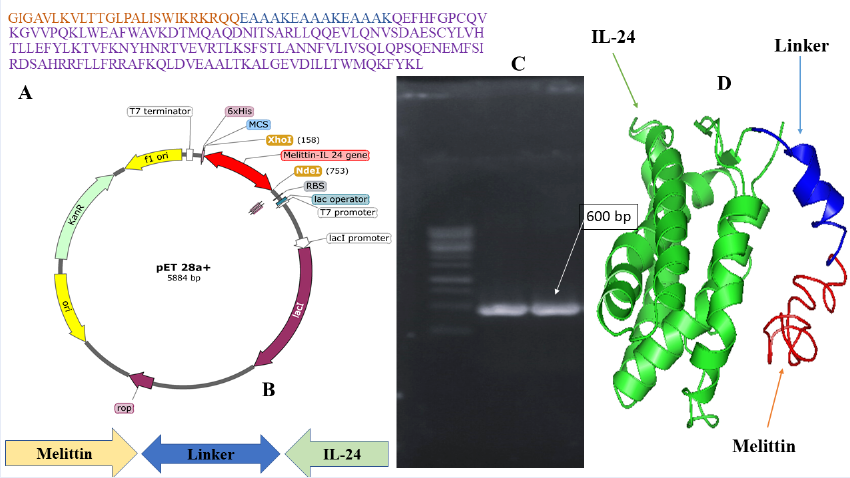
A 465-bp fragment encoding the mature IL-24 peptide was retrieved from the NCBI database and fused to the cytolytic peptide melittin through a rigid (EAAK)₃ linker (Figure 1A). The complete amino-acid sequence of the fusion construct is displayed, highlighting the melittin region, linker, and IL-24 domain. Thirty PCR cycles were run with an annealing temperature of 58 °C using standard cycling parameters (95 C denaturation, 72 C extension), followed by a final 10-min extension at 72 °C. Restriction digestion of the fused gene generated an ≈600-bp fragment, which was confirmed by agarose gel electrophoresis (Figure 1C). Sequence analysis confirmed 99 % identity with the intended sequence. The fragment was ligated into the pET-28a(+) expression vector under the control of the T7 promoter between the NdeI and XhoI sites (Figure 1B). The resulting plasmid was transformed into Escherichia coli BL21(DE3) for expression. To validate structural integrity, 3-D structure prediction with AlphaFold2 indicated proper folding and successful domain fusion (Figure 1D).
Isolation, solubilization, and refolding of IL-24 and melittin–IL-24 inclusion bodies
An BL21(DE3) expression system was used to produce IL-24 and the melittin–IL-24 fusion protein. High-density shake-flask cultures were harvested after induction. Proteins expressed in prokaryotes often fail to fold correctly and accumulate as insoluble inclusion bodies (IBs). The selected clones yielded 10.5 g (L) (IL-24) and 9.5 g (L) (melittin–IL-24) of wet cell biomass. Cell disruption at 15 kpsi followed by centrifugation produced 4.5 g and 3.8 g of IBs, respectively (
Melittin–IL-24 fusion construct. (A) The amino acid sequence of the designed Melittin–linker–IL-24 fusion protein in different colours. (B) Schematic map of the recombinant plasmid pET-28a(+)-Melittin–IL-24 showing the insertion of the fusion gene between the XhoI and NdeI restriction sites under the control of a T7 promoter. (C) Agarose gel electrophoresis confirming successful PCR amplification of the Melittin–IL-24 gene (~600 bp). (D) Predicted 3D structure of fusion protein. The IL-24 domain is colored in green, the linker in blue, and the Melittin domain in red, illustrating the spatial organization of the fusion construct.
|
Designed Construct |
Inclusion bodies (g/L) |
Total protein (mg/L) |
Refolded and Diafilteration (mg/L) |
Purification (mg/L) |
Overall yield (%) |
|
IL-24 |
4.5 ( |
390 ( |
310 ( |
100 ( |
40 ( |
Melittin-IL24 |
3.8 ( |
360 ( |
280 ( |
80 ( |
35 ( |
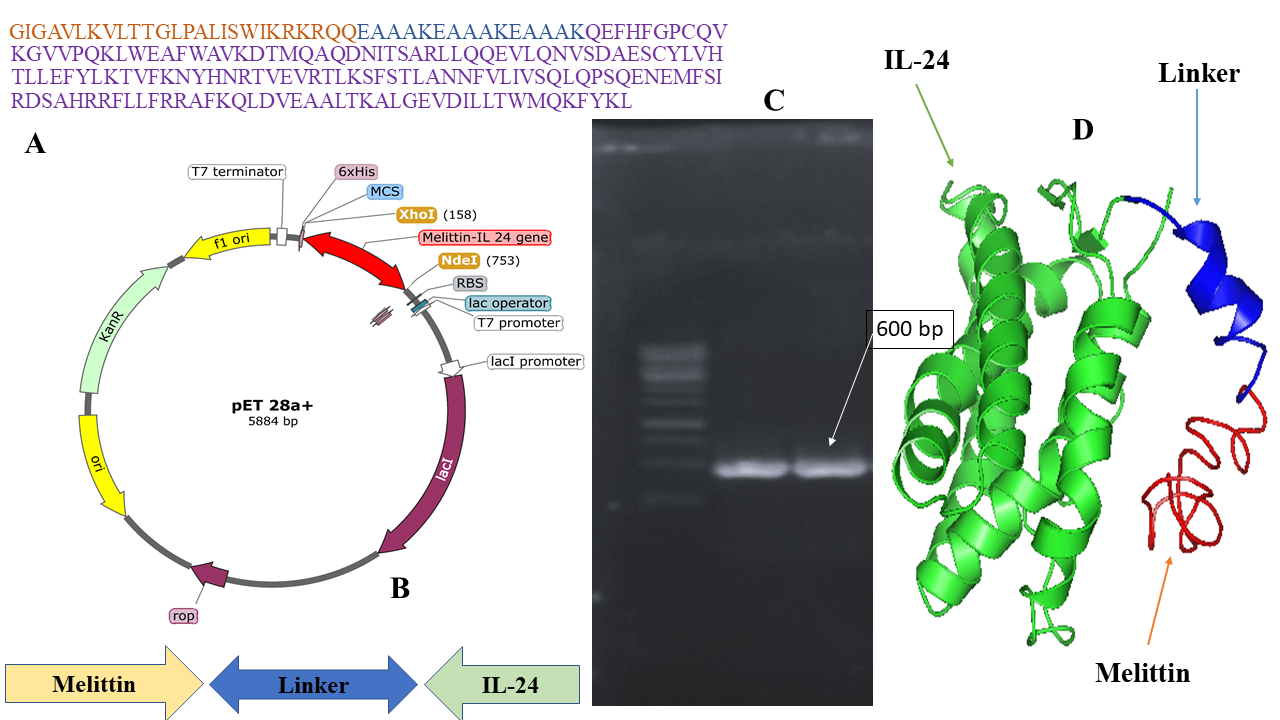
Chromatogram of IL-24 and melittin-IL 24 fusion protein purification through affinity chromatography.
Purification of IL-24 and melittin–IL-24 fusion proteins
Refolded IL-24 and melittin–IL-24 were purified by tangential-flow diafiltration followed by Ni-NTA affinity chromatography. Diafiltration using a 10-kDa-cutoff membrane removed particulates and concentrated the proteins ≈25-fold. The samples were loaded onto a Ni-NTA column on an ÄKTA Explorer (GE Healthcare) system, and proteins were eluted with a single-step gradient to 0.5 M imidazole (Figure 2). Overall yields are summarized in
Characterization of IL-24 and melittin–IL-24 fusion proteins
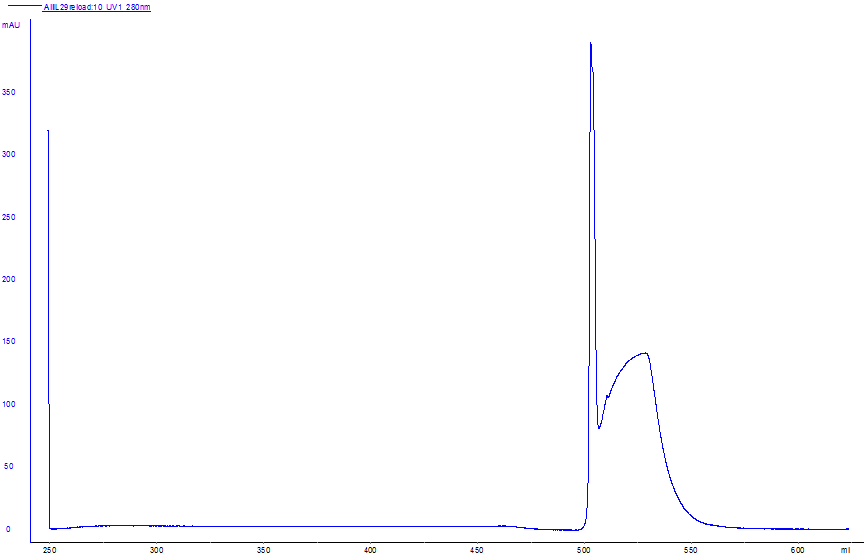
The purity of IL-24 and the melittin–IL-24 fusion protein was confirmed by RP-HPLC, western blotting, and SDS-PAGE (Figure 3A–C). SDS-PAGE (Figure 3C) showed single bands corresponding to IL-24 (≈18 kDa) and melittin–IL-24 (≈22 kDa), indicating >98 % purity. In the western blot (Figure 3B), both proteins reacted with an anti-His₆ monoclonal antibody, confirming their identity. RP-HPLC (Figure 3A) showed a single sharp peak at 19.2 min, consistent with >98 % purity for both proteins.
The purity of IL-24 and the melittin–IL-24 fusion protein was confirmed by RP-HPLC, western blotting, and SDS-PAGE. (A) Analytical RP-HPLC chromatogram of the purified melittin–IL 24 fusion protein indicating a single sharp peak at ~19.2 min, corresponding to >97% purity. (B) Western blot analysis confirming the successful expression of both IL-24 (18.4 kDa) and the Melittin–IL-24 fusion protein (22.4 kDa), using anti-His antibodies. (C) SDS-PAGE analysis of purified protein samples. Lane 1 shows the Melittin–IL-24 fusion protein with a visible band around 22 kDa, and Lane 2 shows the IL-24 control protein. Lane L contains the molecular weight marker. The bands confirm the correct size and purity of the expressed proteins.
Biological activity
The cytotoxicity of IL-24 and melittin–IL-24 was evaluated on MCF-7 breast-cancer cells and normal MCF-10F mammary epithelial cells. Cells were treated with 10–140 µg mL protein for 24 h. MTT assays demonstrated dose-dependent inhibition of MCF-7 viability (Figure 4). Melittin–IL-24 reduced viability to 60 %, 40 %, and 20 % at 30, 50, and 60 µg mL, respectively, whereas IL-24 required 60, 80, and 120 µg mL to reach 80 %, 60 %, and 40 % viability. The IC of melittin–IL-24 was 52 µg mL, approximately two-fold lower than that of IL-24. In MCF-10F cells, only 13.7 % (IL-24) and 21.4 % (melittin–IL-24) were non-viable at 130 µg mL, indicating selective cytotoxicity toward cancer cells. Figure 4 depicts percentage viability for melittin–IL-24 (green) and IL-24 (blue) at the indicated concentrations. These data support a favorable therapeutic window for the melittin–IL-24 fusion protein.
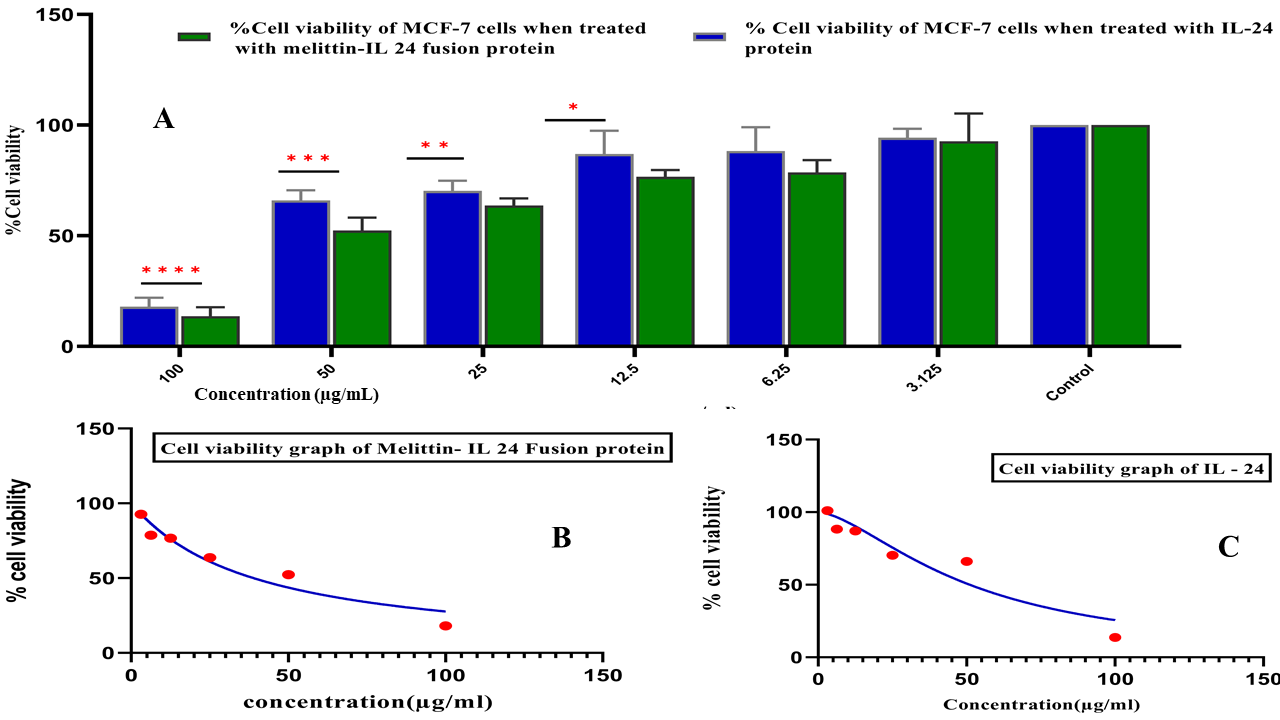
Cytotoxic effects of Melittin–IL-24 fusion protein and IL-24 alone on MCF-7 cells. (A) Bar graph showing % cell viability after 24 h treatment with increasing concentrations (3.125–100 µg/mL). The fusion protein (green) shows greater cytotoxicity than IL-24 (blue). Data are mean ± SD;
Discussion
Breast cancer is among the deadliest cancers worldwide, as highlighted by the World Health Organization30. As the global prevalence of cancer continues to rise and treatment accessibility remains limited, the need for highly specific, targeted drug candidates with potent cytotoxic properties becomes increasingly urgent3132. Fusion proteins, engineered from natural sources, represent a promising class of novel therapeutic agents because they combine functional domains from different proteins, mirroring evolutionary phenomena observed in nature3334. By integrating these domains into a single polypeptide, fusion proteins retain the positive properties of both parent proteins and enhance therapeutic potential. Dimeric fusion proteins, in particular, can exhibit synergistic effects that increase therapeutic efficiency by 10- to 20-fold compared with their monomeric counterparts3536.
Previously, Yang expressed recombinant, non-fused IL-24 in , purified it via chromatography to >96 % purity, and confirmed that it retained cancer-selective, apoptosis-inducing properties after removal of the fusion tag37. However, subsequent studies have fused IL-24 to various peptides to enhance its therapeutic efficacy. For instance, Xiao . designed an IL-24–RGD peptide fusion that significantly inhibited MCF-7 tumor growth by inducing apoptosis38. Jahanian-Najafabadi introduced P28-IL-24, a chimeric protein that demonstrated substantial cytotoxicity and tumor reduction, primarily through apoptosis39. Zhang engineered TAT-IL-24-KDEL, which selectively inhibited several cancer cell lines by inducing ER-stress-mediated apoptosis while sparing normal cells, showing effectiveness both and 40. Pourhadi produced IL-24-BR2, which exhibited significant cytotoxic effects on MCF-7 breast-cancer cells, unlike IL-24 alone, underscoring its potential for targeted therapy41.
In the present study we constructed a novel fusion protein by combining Interleukin-24 (IL-24) with melittin, a cytolytic peptide derived from bee venom, to assess its therapeutic potential against breast cancer. The fusion gene was assembled with recombinant-DNA techniques, joining the individual genes encoding IL-24 and melittin into a single open reading frame, and expressed in . The resulting protein, designated melittin–IL-24, represents a bifunctional molecule with potential applications in cancer therapy.
Our previous investigations predicted that the melittin–IL-24 fusion protein possesses promising anti-breast-cancer activity: computational modeling suggested that the construct could enhance therapeutic efficacy by combining the selective tumor-targeting capability of IL-24 with the potent cytotoxic effects of melittin26. These findings provided the rationale for subsequent evaluation. The experimental results indicate that this bifunctional protein operates through a dual mechanism: IL-24 contributes to tumor-selective apoptosis and immune modulation, whereas melittin exerts direct cytolytic effects on cancer cells. Collectively, the data show that melittin–IL-24 exhibits potent cytolytic activity against breast-cancer cells while maintaining high specificity.
The cytotoxic effects observed in MCF-7 cells, coupled with minimal toxicity in MCF-10F normal epithelial cells, indicate a promising therapeutic window for this fusion protein. Affinity chromatography on nickel–agarose resin, exploiting a C-terminal hexahistidine tag, enabled efficient purification42. Melittin–IL-24 reduced MCF-7 viability in a dose-dependent manner, with an IC of 66 µg mL⁻¹; by contrast, IL-24 alone had an IC of 52 µg mL. Importantly, cytotoxicity toward normal MCF-10F cells remained minimal even at the highest concentrations tested, supporting the construct’s selectivity. Across all tested concentrations, IL-24 alone decreased cell viability, confirming its anti-cancer potential. At 50 and 100 µg mL, IL-24 significantly reduced viability relative to untreated controls, demonstrating a clear dose-dependent effect. The melittin–IL-24 fusion protein further decreased cell viability, implying a synergistic interaction between melittin and IL-24.
Conclusions
This study presents an innovative cancer-therapy strategy based on a melittin–IL-24 fusion protein that combines the anti-cancer properties of IL-24 with the potent cell-lytic activity of melittin. Its rational design, efficient expression, and subsequent purification underscore the fusion protein’s potential as a targeted therapeutic. Fusion proteins can offer higher potency, greater specificity, and simultaneous interference with multiple pathways that drive tumor growth and survival. Although the melittin–IL-24 construct showed promising in-vitro activity against breast-cancer cells, several limitations persist. The work was confined to a single breast-cancer cell line, and in-vivo validation as well as detailed pharmacokinetic profiling are still required to establish safety and stability. Moreover, the study lacked mechanistic assays—such as apoptosis measurements or analyses of oxidative and endoplasmic-reticulum stress—that could clarify the pathways responsible for the observed cytotoxicity.
Abbreviations
6×His/His₆: Six-Histidine tag / Hexahistidine tag; ANOVA: Analysis of Variance; ATCC: American Type Culture Collection; bp: Base Pairs; BR2: A cell-penetrating peptide; DMEM: Dulbecco's Modified Eagle Medium; DMSO: Dimethyl Sulfoxide; EAAAKEAAAKEAAAK: A rigid protein linker sequence (often called an (EAAK)₃ linker); EDTA: Ethylenediaminetetraacetic acid; ER: Endoplasmic Reticulum; FBS: Fetal Bovine Serum; Fc: Fragment, crystallizable region; GSH: Reduced Glutathione; GSSG: Oxidized Glutathione; HPLC: High-Performance Liquid Chromatography; HRP: Horseradish Peroxidase; HSD: Honestly Significant Difference; IBs: Inclusion Bodies; IC₅₀: Half Maximal Inhibitory Concentration; IL-24: Interleukin-24; IPTG: Isopropyl β-d-1-thiogalactopyranoside; KDEL: Lys-Asp-Glu-Leu; kDa: Kilodalton; LB: Lysogeny Broth; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide; NCBI: National Center for Biotechnology Information; NBT/BCIP: Nitro-Blue Tetrazolium / 5-Bromo-4-Chloro-3'-Indolyphosphate; Ni-NTA: Nickel-Nitrilotriacetic Acid; OD₆₀₀: Optical Density at 600 nm; P28: A tumor-targeting peptide; PCR: Polymerase Chain Reaction; pET-28: A common protein expression vector; PMSF: Phenylmethylsulfonyl Fluoride; pUC57: A common cloning vector; PVDF: Polyvinylidene Fluoride; RGD: Arginine-Glycine-Aspartic acid; RP-HPLC: Reversed-Phase High-Performance Liquid Chromatography; rpm: Revolutions Per Minute; SD: Standard Deviation; SDS-PAGE: Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis; TAT: Trans-Activator of Transcription; TFA: Trifluoroacetic Acid; Tris-HCl: Tris(hydroxymethyl)aminomethane hydrochloride
Acknowledgments
The authors are thankful to the University of the Punjab and HEC Pakistan for providing access to the necessary instrumentation and research facilities that enabled the successful execution of this study
Author’s contributions
Formal analysis. methodology, data curation, validation: HMR: draft preparation, critical revision & final editing of manuscript: HB. All authors read and approved the final manuscript.
Funding
The current project was funded by HEC Pakistan through NRPU 2021 (Project No. 16935) and University of the Punjab, Lahore, Pakistan.
Availability of data and materials
Data is accessible upon inquiry from Hamid Bashir via Email: hamid.camb@pu.edu.pk.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

